Recombinant Human Syndecan-1 Fc Chimera Avi-tag Protein, CF
R&D Systems, part of Bio-Techne | Catalog # AVI10913

Key Product Details
Source
Accession #
Structure / Form
Conjugate
Applications
Product Specifications
Source
| Human Syndecan-1 (Gln18-Gln251) Accession # AAH08765.1 |
IEGRMD | Human IgG1 Fc (Pro100-Lys330) |
Avi-tag |
| N-terminus | C-terminus | ||
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
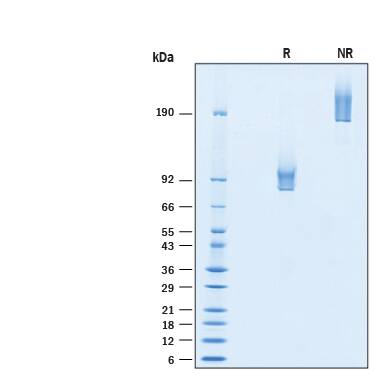
SDS-PAGE
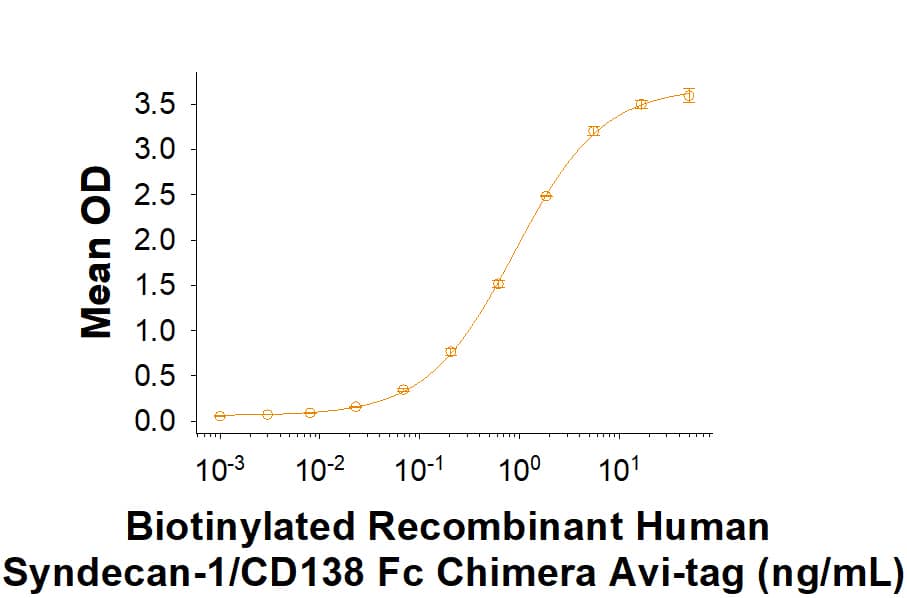
Activity
Biotinylated Recombinant Human Syndecan-1/CD138 Fc Chimera Avi-tag (Catalog # AVI10913) binds to Human Syndecan-1/CD138 Antibody (Catalog # MAB2780) with an ED50 of 0.400-5.00 ng/mL.
Measured by the ability of the immobilized protein to support the adhesion of Saos-2 human osteosarcoma cells. The ED50 for this effect is 0.5-6.0 µg/mL.
Scientific Data Images for Recombinant Human Syndecan-1 Fc Chimera Avi-tag Protein, CF
Biotinylated Recombinant Human Syndecan‑1/CD138 Fc Chimera Avi-tag Protein Binding Activity.
In a functional ELISA, Biotinylated Recombinant Human Syndecan-1/CD138 Fc Chimera Avi-tag (Catalog # AVI10913) binds to Human Syndecan-1/CD138 Antibody (Catalog # MAB2780) with an ED50 of 0.400-5.00 ng/mL.Biotinylated Recombinant Human Syndecan‑1/CD138 Fc Chimera Avi-tag Protein SDS-PAGE.
2 μg/lane of Biotinylated Recombinant Human Syndecan‑1/CD138 Fc Chimera Avi-tag Protein (Catalog # AVI10913) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 81-105 kDa and 160-210 kDa, respectively.Formulation, Preparation and Storage
AVI10913
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 500 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: Syndecan-1/CD138
Syndecan-1, designated CD138, is a dimeric type I transmembrane (TM) protein that belongs to the syndecan family of Type 1 transmembrane proteins (1, 2). The four syndecan family members are major carriers of heparan sulfate (HS) and chondroitin sulfate glycosaminoglycans (GAGs) that have different expression patterns and extracellular sequences. Syndecan-1 forms weak non-covalent homodimers, or heterodimers with Syndecan-2 or -3, through interactions of the transmembrane domain (3). It is synthesized as a 310 amino acid (aa) precursor with a 17 aa signal sequence, a 234 aa extracellular domain (ECD) that includes three closely-spaced consensus Ser-Gly HS attachment sites near the N-terminus, a 25 aa TM segment, and a 34 aa cytoplasmic region that includes a PDZ binding motif with a tyrosine phosphorylation site. The ECD is variably modified by GAGs, producing molecular weights of 120-200 kDa for native Syndecan-1. Soluble forms are shed via proteolytic cleavage. Human Syndecan-1 ECD shares 65-71% aa identity with the ECD of rat, mouse, canine, equine and bovine Syndecan-1. Syndecan-1 shows highest expression on epithelial cells such as keratinocytes, and terminally differentiated B cells such as plasma cells (4, 5). It aids wound healing in skin, cornea, and heart following myocardial infarction by promoting re-epithelialization, migration, and collagen deposition (4-8). It binds chemokines, creating chemotactic gradients when shed, but also binds and modulates integrins to control the influx of leukocytes (5, 7, 9). The net effect is to allow, but limit, inflammation. In myeloma and other cancers, shedding of Syndecan-1 can facilitate growth, angiogenesis and metastasis (10-12). Growth factors, such as FGFs and HGF, bind GAG chains and use Syndecan-1 as a coreceptor (12, 13). The GAG chains may also be used by a variety of viruses and bacteria for cell adhesion and uptake (4). Our Avi-tag Biotinylated human Syndecan-1/CD138 features biotinylation at a single site contained within the Avi-tag, a unique 15 amino acid peptide. Protein orientation will be uniform when bound to streptavidin-coated surface due to the precise control of biotinylation and the rest of the protein is unchanged so there is no interference in the protein's bioactivity.
References
- Tkachenko, E. et al. (2005) Circ. Res. 96:488.
- Mali, M. et al. (1990) J. Biol. Chem. 265:6884.
- Dews, I.C. and K.R. MacKenzie (2007) Proc. Natl. Acad. Sci. USA 104:20782.
- Fears, C.Y. and A. Woods (2006) Matrix Biol. 25:443.
- Stepp, M.A. et al. (2002) J. Cell Sci. 115:4517.
- Ojeh, N. et al. (2008) J. Invest. Dermatol. 128:26.
- Stepp, M.A. et al. (2007) J. Cell Sci. 120:2851.
- Vanhoutte, D. et al. (2007) Circulation 115:475.
- Li, Q. et al. (2002) Cell 111:635.
- Beauvais, D.M. et al. (2009) J. Exp. Med. 206:691.
- Yang, Y. et al. (2007) J. Biol. Chem. 282:13326.
- Derksen, P.W.B. et al. (2002) Blood 99:1405.
- Su, G. et al. (2007) J. Biol. Chem. 282:14906.
Alternate Names
Gene Symbol
UniProt
Additional Syndecan-1/CD138 Products
Product Documents for Recombinant Human Syndecan-1 Fc Chimera Avi-tag Protein, CF
Product Specific Notices for Recombinant Human Syndecan-1 Fc Chimera Avi-tag Protein, CF
For research use only