Recombinant Human TRANCE/RANK L His Avi-tag Protein, CF
R&D Systems, part of Bio-Techne | Catalog # AVI390

Key Product Details
Source
Accession #
Structure / Form
Conjugate
Applications
Product Specifications
Source
| Avi-tag | HHHHHH | Human TRANCe/TNFSF11/RANK L (Ile140-Asp317) Accession # O14788.1 |
| N-terminus | C-terminus |
Purity
Endotoxin Level
N-terminal Sequence Analysis
Gly of Avi-tag
Predicted Molecular Mass
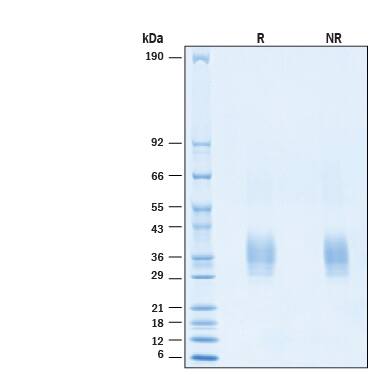
SDS-PAGE
Activity
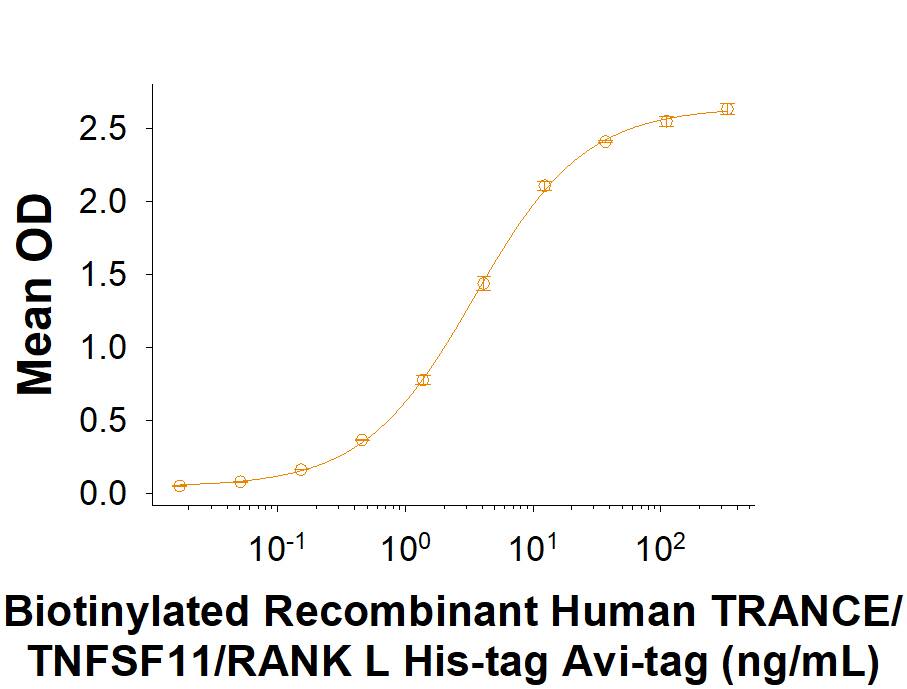
When Recombinant Human RANK/TNFRSF11A Fc Chimera Protein (Catalog # 683-RK) is coated at 0.5 μg/mL (100 μL/well), the concentration of Biotinylated Recombinant Human TRANCE/TNFSF11/RANK L His-tag Avi-tag that produces 50% optimal binding response is 3.00-15.0 ng/mL
Scientific Data Images for Recombinant Human TRANCE/RANK L His Avi-tag Protein, CF
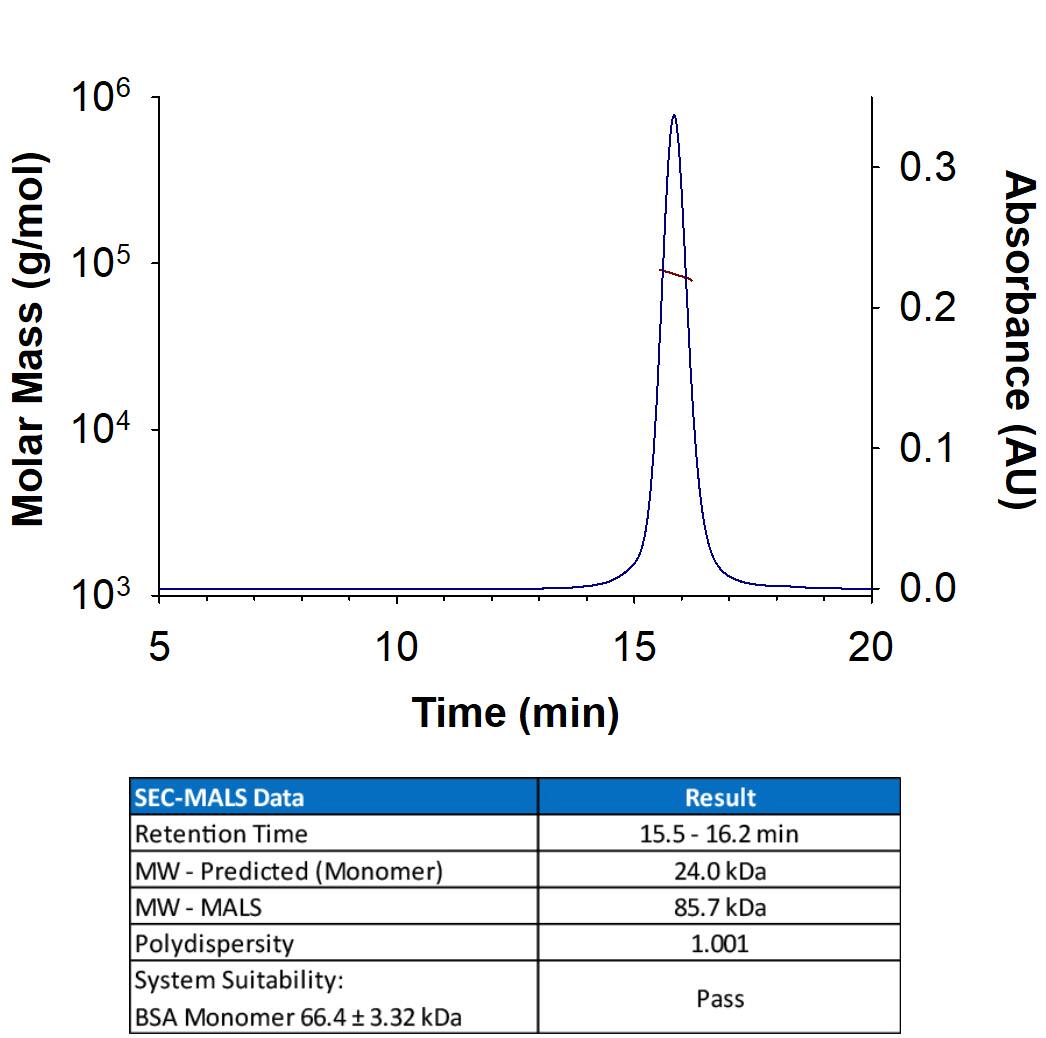
Biotinylated Recombinant Human TRANCE/TNFSF11/RANK L His-tag Avi-tag Protein SEC-MALS.
Recombinant Human TRANCE/RANK L/TNFSF11 (Catalog # AVI390) has a molecular weight (MW) of 85.7 kDa as analyzed by SEC-MALS, suggesting that this protein is a homotrimer. MW may differ from predicted MW due to post-translational modifications (PTMs) present (i.e. Glycosylation).Biotinylated Recombinant Human TRANCE/TNFSF11/RANK L His-tag Avi-tag Protein SDS-PAGE.
2 μg/lane of Biotinylated Recombinant Human TRANCE/TNFSF11/RANK L His-tag Avi-tag Protein (Catalog # AVI390) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 26-39 kDa.Biotinylated Recombinant Human TRANCE/RANK L His-tag Avi-tag Protein Binding Activity.
When Recombinant Human RANK/TNFRSF11A Fc Chimera Protein (683-RK) is coated at 0.5 μg/mL (100 μL/well), the concentration of Biotinylated Recombinant Human TRANCE/TNFSF11/RANK L His-tag Avi-tag (Catalog # AVI390) that produces 50% optimal binding response is 3.00‑15.0 ng/mL.Formulation, Preparation and Storage
AVI390
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 100 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: TRANCE/TNFSF11/RANK L
RANK L (receptor activator of NF-kappa B ligand), also called TRANCE (TNF-related activation-induced cytokines), OPGL (osteoprotegerin ligand), or ODF (osteoclast differentiation factor), is a 39‑45 kDa type II transmembrane (TM) protein in the tumor necrosis factor family, designated TNFSF11 (1‑5). RANK L, produced by osteoblasts and bone marrow stromal cells, is required for differentiation of osteoclasts and stimulates bone resorption (4, 6). It is also produced by activated T cells and augments dendritic cell stimulation; RANK L-/- mice lack lymph nodes and have impaired thymocyte development (1‑3, 6). The human RANK L cDNA encodes 317 amino acids (aa), including a 47 aa cytoplasmic domain, a 21 aa TM region, and a 249 aa extracellular domain (ECD) with two potential N‑linked glycosylation sites (note: Arg85‑Asp245 of Accession # AAC51762 is identical to Arg157‑Asp317 of SwissProt # O14788. This aa range contains the ECD trimerization and receptor‑binding motifs, but not ECD proteolytic cleavage sites). Within the ECD, human RANK L shares 89%, 89%, 93% and 95% aa identity with mouse, rat, bovine and porcine RANK L, respectively. Mouse RANK L can stimulate human osteoclast differentiation (4). Like most TNF family members, RANK L can form trimers (1). Soluble 31, 25 and 24 kDa forms of RANK L can be created by usage of alternate start sites at aa 74 or 146, or proteolytic cleavage by osteoblast- or stromal cell‑derived ADAM10 (after aa 139) or MMP14 (aa 146), or bone metastatic prostate tumor-derived MT1-MMP (aa 146) (5, 7, 8). Both TM and soluble extracellular RANK L act by engaging RANK receptors and are antagonized by the decoy receptor, OPG (osteoprotegrin) (2, 5). In resting cells, the majority of RANK L is stored in secretory lysosomes (9). In mammary epithelia, RANK L is upregulated by pregnancy hormones and is essential for the formation of a lactating mammary gland (10). In the brain, astrocyte RANK L mediates body temperature regulation (11). Pathologically, RANK L is thought to mediate post-menopausal osteoporosis, vascular calcification, progestin-induced breast cancer, cancer-induced bone disease, and osteopetrosis (in RANK L deficiencies) (12‑16). Our Avi-tag Biotinylated human TRANCE/RANK L features biotinylation at a single site contained within the Avi-tag, a unique 15 amino acid peptide. Protein orientation will be uniform when bound to streptavidin-coated surface due to the precise control of biotinylation and the rest of the protein is unchanged so there is no interference in the protein's bioactivity.
References
- Leibbrandt, A. and J.M. Penninger (2008) Ann. N.Y. Acad. Sci. 1143:123.
- Wong, B.R. et al. (1997) J. Biol. Chem. 272:25190.
- Anderson, D.M. et al. (1997) Nature 390:175.
- Lacey, D.L. et al. (1998) Cell 93:165.
- Hikita, A. et al. (2006) J. Biol. Chem. 281:36846.
- Kong, Y-Y. et al. (1999) Nature 397:315.
- Accession # NP_143026 and EAX08679.
- Sabbota, A.L. et al. (2010) Cancer Res. 70:5558.
- Aoki, S. et al. (2010) J. Bone Miner. Res. 25:1907.
- Fata, J.E. et al. (2000) Cell 103:41.
- Hanada, R. et al. (2009) Nature 426:505.
- Osako, M.K. et al. (2010) Circ. Res. 107:466.
- Schramek, D. et al. (2010) Nature 468:98.
- Gonzalez-Suarez, E. et al. (2010) Nature 468:103.
- Dougall, W.C. and M. Chaisson (2006) Cancer Metastasis Rev. 25:541.
- Sobacchi, C. et al. (2007) Nat. Genet. 39:960.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional TRANCE/TNFSF11/RANK L Products
Product Documents for Recombinant Human TRANCE/RANK L His Avi-tag Protein, CF
Product Specific Notices for Recombinant Human TRANCE/RANK L His Avi-tag Protein, CF
For research use only