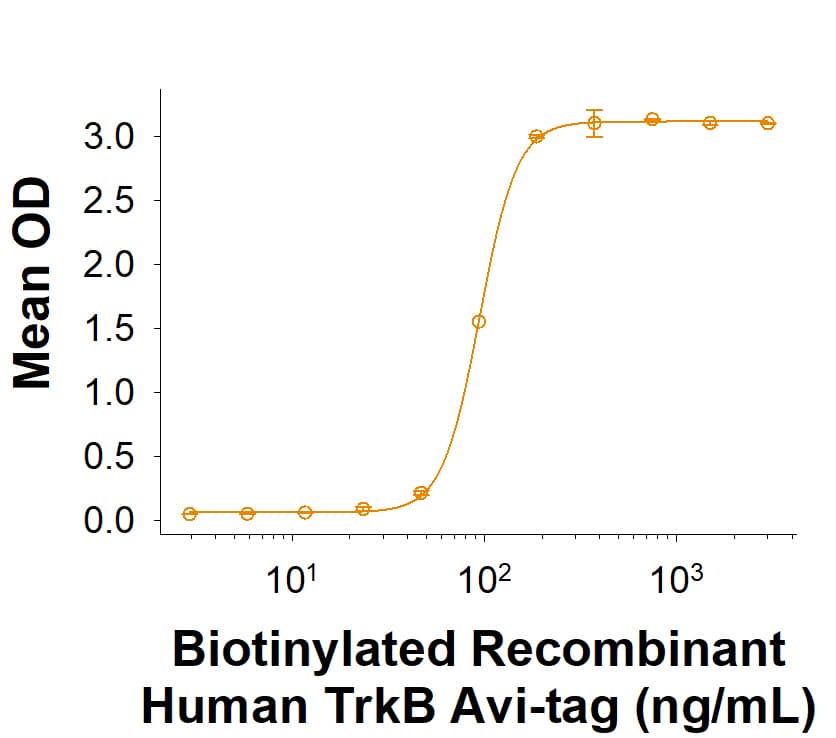
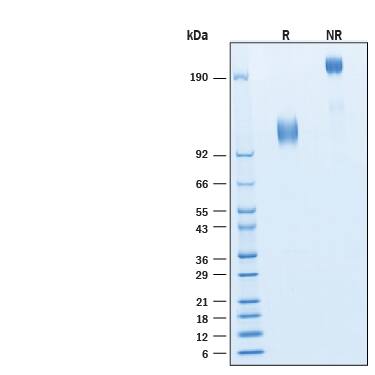
The neurotrophins, including NGF, BDNF, NT-3, and NT-4/5 constitute a group of structurally related, secreted proteins that play an important role in the development and function of the nervous system. The biological activities of the neurotrophins are mediated by binding to the different members of the Trk family tyrosine kinase receptors. Three Trk family proteins, TrkA, TrkB, and TrkC, exhibiting different ligand specificities, have been identified. TrkA binds NGF, TrkB binds BDNF and NT‑4/5 and TrkC binds NT-3. All Trk family proteins share a conserved complex subdomain organization consisting of a signal peptide, two cysteine-rich domains, a cluster of three leucine-rich motifs, and two immunoglobulin-like domains in the extracellular region, as well as an intracellular region that contains the tyrosine kinase domain. Natural splice variants of the different Trks, including TrkB variants lacking the first cysteine-rich domain, the first and second or all three of the leucine-rich motifs, or the tyrosine kinase domain, have been described. The role of the different extracellular subdomains of TrkB in mediating neurotrophin binding and discrimination is currently being investigated. At the protein sequence level, human and rat TrkB are greater than 90% identical and the proteins exhibit cross-species activity. TrkB is primarily expressed in the nervous system. However, low levels of TrkB expression have also been observed in a wide variety of tissues (pancreas, kidneys, ovary) outside the nervous system. Our Avi-tag Biotinylated human TrkB features biotinylation at a single site contained within the Avi-tag, a unique 15 amino acid peptide. Protein orientation will be uniform when bound to streptavidin-coated surface due to the precise control of biotinylation and the rest of the protein is unchanged so there is no interference in the protein's bioactivity.