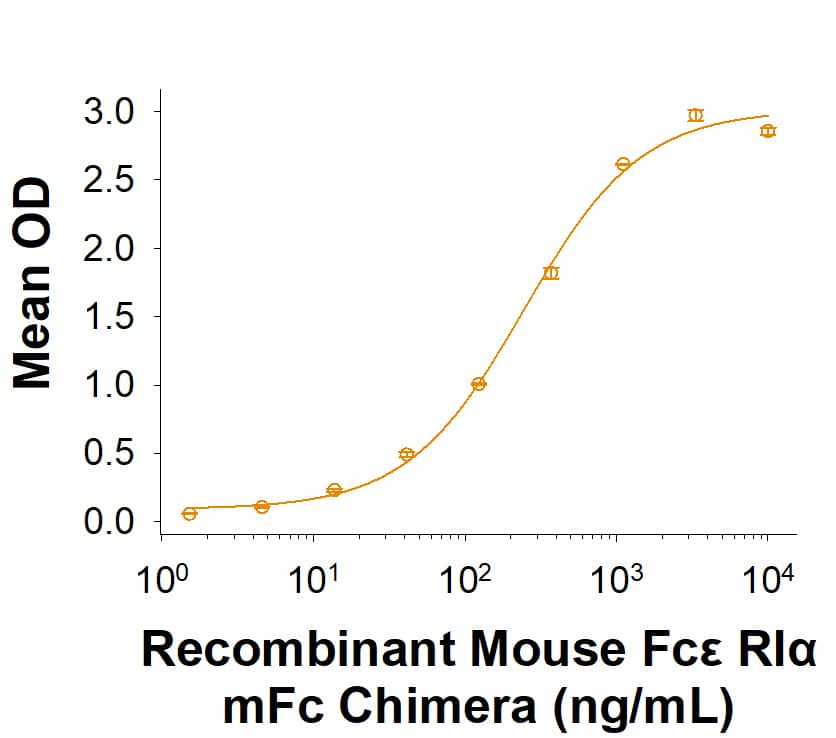
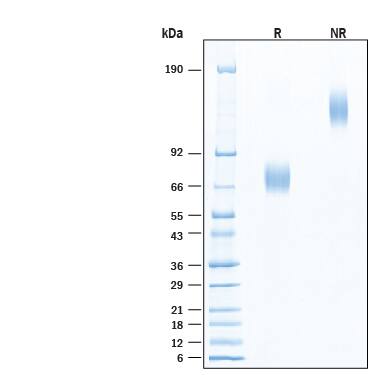
The alpha subunit of the high affinity IgE receptor (Fc epsilon RI alpha or Fc epsilon RIA) is an IgE‑binding type I transmembrane glycoprotein of the multichain immune recognition (MIRR) family (1, 2). The receptor, Fc epsilon RI, is a tetrameric complex of one alpha, one beta and two gamma subunits ( alpha beta gamma 2) on mast cells and basophils (1). An alternate trimeric form ( alpha gamma 2) is expressed on human, but not rodent, mast cells, basophils, eosinophils and professional antigen presenting cells (3). While the gamma subunit is essential for expression of Fc epsilon RI alpha on the cell surface and for cell signaling, the beta subunit, when present, increases the halflife of the Fc epsilon RI complex on the cell surface (3, 4). An isoform of the beta subunit, beta T, blocks processing of the alpha subunit and its cell surface expression (2, 3, 5). Mouse Fc epsilon RI alpha cDNA encodes 250 amino acids (aa) including a 23 aa signal sequence, a 181 aa extracellular domain containing two Ig‑like domains, a 19 aa transmembrane domain and a 27 aa cytoplasmic sequence. Mouse Fc epsilon RI alpha shares 52% and 71% aa sequence identity with human and rat Fc epsilon RI alpha, respectively. Binding of IgE alone increases surface expression of Fc epsilon RI, while crosslinking of IgE/Fc epsilon RI complexes by IgE ligands (allergens) initiates receptor internalization and signaling (2, 4, 5). Mast cell and basophil activation by IgE/Fc epsilon RI crosslinking causes degranulation, releasing histamine, leukotrienes, prostaglandins, and other mediators of immediate‑type and late‑phase allergic reactions. Circulating autoantibodies that crosslink Fc epsilon RI alpha are often found in patients with chronic urticaria (6). Fc epsilon RI on human antigen presenting cells mediates uptake and processing of allergens for presentation by class II MHC (2, 3). Fc epsilon RI expression on human DC and Langerhans cells is up‑regulated during allergic reactions (atopy) and correlates with serum IgE concentration (3).