Recombinant Mouse LIX (aa 49-118) Protein
R&D Systems, part of Bio-Techne | Catalog # 9194-MC

Key Product Details
Product Specifications
Source
Thr49-Ala118
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
SDS-PAGE
Activity
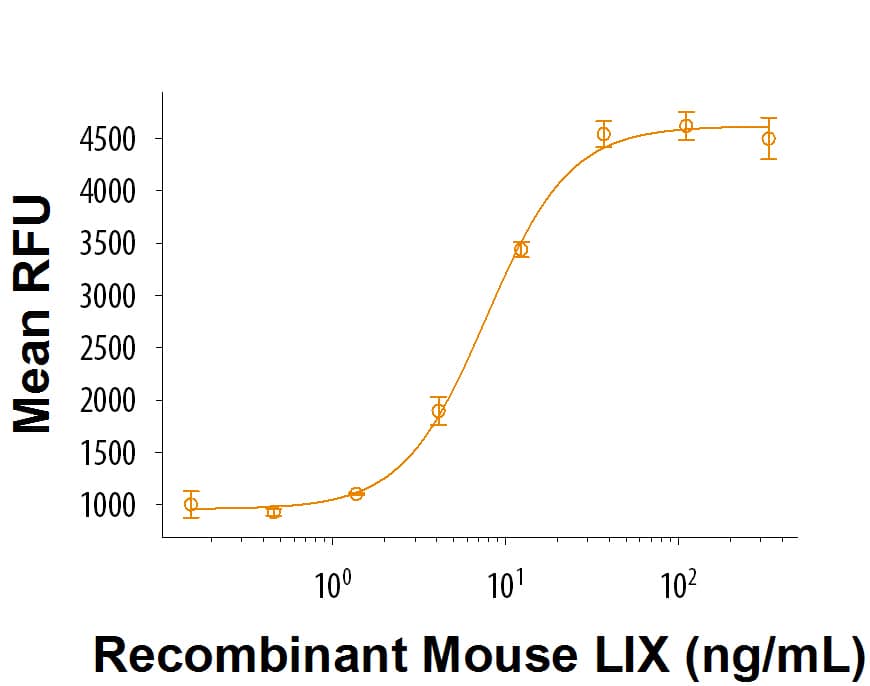
The ED50 for this effect is 1.5-9 ng/mL.
Scientific Data Images for Recombinant Mouse LIX (aa 49-118) Protein
Recombinant Mouse LIX (aa 49-118) Protein Bioactivity
Recombinant Mouse LIX (Catalog # 9194-MC) attracts BaF3 mouse pro B cells transfected with human CXCR2. The ED50 for this effect is 1.5-9 ng/mL.Formulation, Preparation and Storage
Carrier Free
What does CF mean?CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
What formulation is right for me?In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
Carrier: 9194-MC
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 250 μg/mL in PBS containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Carrier Free: 9194-MC/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS. |
| Reconstitution | Reconstitute at 250 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: LIX
LIX (Liposaccharide-Induced CXC chemokine; also GARG-8) is a secreted 8-10 kDa member of the ELR+ class, CXC family, chemokine superfamily of molecules
(1-4). It is one of five CXC chemokines in mouse, and serves as a functional equivalent of human ENA-78 and GCP-2. Mouse LIX is synthesized as a 132 amino acid (aa) precursor that contains an extended 40 aa signal sequence plus a 92 aa mature peptide (aa 41-132) (1, 5). No glycosylation has been reported for LIX. Depending upon the author, the 92 aa full-length isoform is typically referred to as either simply LIX, or LIX (1-92). Shorter isoforms have an accompanying range designation. Although LIX has (marginal) activity, proteolytic processing is necessary for full bioactivity (5, 6). To date, over 25 enzymatically-generated isoforms have been experimentally noted, ranging from 69-92 aa in length (6). These may show only N-terminal, C-terminal, or dual-end processing, with removal of up to 10 N-terminal amino acids (# 41-48) and 13 or 14 C-terminal amino acids (# 119-132). In vivo, it appears that multiple MMPs act sequentially (and redundantly) to generate a "standard" LIX isoform that consists of aa 5-78 of the mature form. It is suggested that initially, MMP-2 is induced locally, and cleaves LIX 1-92 between Ser4-Val5. This truncation results in an active product (LIX [5-92]) that chemoattracts PMNs. Newly arriving PMNs now secrete MMP-9, which performs the same cleavage as MMP-2, chemoattracting more PMNs. Neutrophil-derived MMP-8 is secreted next, which cleaves both the Ser4-Val5 and Lys79-Arg80 bonds, creating LIX (5-79) (7, 8). Although somewhat unclear, macrophage derived MMP-12 also contributes to LIX activation, and in some circumstances cleaves the ELR motif of activated chemokines (but not LIX), rendering them inactive and downmodulating the inflammatory response (9). Over aa 41-119 of the precursor, mouse LIX shares 73% and 63% aa sequence identity with rat LIX and human GCP-2, respectively. Mouse LIX is known to be active on human cells (6). Cells known to express LIX vary widely and include oligodendroglia (10) adipose tissue stem cells and macrophages (11, 12) platelets and endothelial cells (13) colonic epithelium (14) cardiomyocytes (15) Type II greater alveolar cells and ileal enterocytes (16) hepatocytes (17) and fibroblasts from multiple tissues (18, 19, 20).
LIX is both constitutively expressed (16) and induced in response to a variety of stimuli, including LPS (18, 21) TNF-alpha (22) mast cell protease-6 (19) leptin (14) and oncostatin M (20). Circulating LIX has two known signaling receptors, CXCR2 and CXCR1. Although the former is considered most important, the latter may prove to have nonredundant functions (6, 21, 23). LIX also binds to DARC, a non-signaling receptor prominently expressed on erythrocytes. Although non-signaling, this receptor serves as a sink or depot for LIX, keeping it from activating CXCR1 and CXCR2 (24). Functionally, LIX is best known as a chemoattractant for neutrophils (5, 9, 15). But it also reportedly chemoattracts macrophages and induces production of NO (14, 25) regulates gut IL-17 and G-CSF secretion (16) promotes TNF-alpha expression from mast cells and macrophages (26) and induces neurite outgrowth and protects against neuronal apoptosis by serving as an atypical growth factor (10).
References
- Hall-Glenn, F. and K.M. Lyons (2011) Cell. Mol. Life Sci. 68:3209.
- Bradham, D.M. et al. (1991) J. Cell Biol. 114:1285.
- Gao, R. and D.R. Brigstock (2004) J. Biol. Chem. 279:8848.
- Schober, J.M. et al. (2002) Blood 99:4457.
- Heng, E.C.K. et al. (2006) J. Cell Biochem. 98:409.
- Jedsadayanmata, A. et al. (1999) J. Biol. Chem. 274:24321.
- Gao, R. et al. (2003) Hepatol. Res. 27:214.
- Mercurio, S. et al. (2004) Development 131:2137.
- Wahab, N.A. et al. (2005) J. Am. Soc. Nephrol. 16:340.
- Abreu, J.G. et al. (2002) Nat. Cell Biol. 4:599.
- Hashimoto, G. et al. (2002) J. Biol. Chem. 277:36288.
- Khodosevich, K. et al. (2013) Neuron 79:1136.
- Shi-Wen, X. et al. (2006) J. Biol. Chem. 281:10715.
- Lee, C.H. et al. (2010) J. Clin. Invest. 120:3340.
- Canalis, E. et al. (2010) Endocrinology 151:3490.
- Nakanishi, T. et al. (2000) Endocrinology 141:264.
- Ivkovic, S. et al. (2003) Development 130:2779.
- Hall-Glenn, F. et al. (2012) PLoS One 7:e30562.
- Shimo, T. et al. (1999) J. Biochem. 126:137.
Alternate Names
Gene Symbol
UniProt
Additional LIX Products
Product Documents for Recombinant Mouse LIX (aa 49-118) Protein
Product Specific Notices for Recombinant Mouse LIX (aa 49-118) Protein
For research use only