Interferons: Cytokines with Anti-Viral and Immunomodulatory Properties
Type I Interferons

Type I Interferon Signaling Pathways
Type I Interferons (IFNs)
Type I interferons play an important role in inhibiting viral replication and orchestrating anti-viral innate immune responses. More recently, they have been reported to be involved in the host response to bacterial, fungal, and parasitic infections as well. The type I interferon family includes 13 IFN-alpha subtypes in humans (14 in mice), IFN-beta, IFN-delta, IFN-epsilon, IFN-kappa, IFN-tau, IFN-omega, and IFN-zeta/Limitin, although IFN-delta, IFN-tau, and IFN-zeta have only been described in pigs, cattle, and mice, respectively. Despite considerable sequence diversity among the type I interferons, they all share a similar four alpha-helix bundle structure characteristic of other helical cytokines, that is arranged in an up-up-down-down topology with an extra alpha helix.
Cellular Sources of Type I IFNs
Type I interferons are produced by multiple cell types in response to activation of cell surface and intracellular pattern recognition receptors, including TLR3, TLR4, TLR7, TLR8, TLR9, and RIG-I. These receptors activate intracellular signaling pathways that result in the phosphorylation and activation of IRF3 and IRF7, two transcription factors that homodimerize and translocate to the nucleus where they are involved in inducing the expression of type I interferons. Although plasmacytoid dendritic cells (pDCs) were originally thought to be the primary producers of type I interferons, macrophages, conventional dendritic cells (cDCs), and inflammatory monocytes have also been shown to be significant cellular sources.
Type I IFN Signaling
Following secretion, all type I interferons signal through a heterodimeric receptor complex composed of IFN-alpha/beta RI and IFN-alpha/beta R2, which are expressed on most cell types. Ligand binding to the type I IFN receptor activates Jak-STAT signaling, resulting in the STAT-induced expression of numerous target genes. Phosphorylated STAT1, STAT2, and IRF9 form a complex known as the IFN-stimulated gene factor 3 (ISGF3) complex, which binds to IFN-stimulated response elements (ISREs) located in the promoter regions of multiple IFN-stimulated genes. Other STAT dimers regulate gene expression by binding to IFN-gamma-activated sequence sites. In addition to the Jak-STAT signaling pathway, type I interferons activate the MAPK, PI 3-K-Akt, and NF-kappa B signaling pathways. Together, these pathways induce the expression of multiple pro- or anti-apoptotic molecules, cytokines, and chemokines, which mediate the anti-viral and anti-proliferative effects of type I interferons. Additionally, type I IFNs can regulate the activities of a variety of immune cell types. They promote the activation, survival, and cytotoxic functions of natural killer cells, enhance dendritic cell functions by promoting the expression of MHC and co-stimulatory molecules, and can directly regulate T cell activation, proliferation, and cytokine secretion.
Bio-Techne offers a large selection of recombinant interferons and viral IFN inhibitors for studying the effects of these proteins on different cell types. We also offer single and multianalyte immunoassays in multiple different formats to make it easy to monitor immune responses, and antibodies for IFNs, IFN receptors, and intracellular signaling molecules that are validated for one or more of the following applications: blocking/neutralization, Western blot, flow cytometry, and immunohistochemistry.
Type I Interferons - Products by Molecule
Type I Interferon Receptors - Products by Molecule
Type I Interferon Inhibitors - Products by Molecule
Anti-Viral Activity Mediated by R&D Systems Recombinant Human IFN-alpha 2 and Inhibition by the Type I IFN inhibitor, Recombinant Viral B18R

Viral B18R Inhibits Type I IFN-mediated Anti-Viral Activity. The HeLa human cervical epithelial cell line infected with encephalomyocarditis virus (EMCV) was treated with increasing concentrations of Recombinant Human IFN-alpha 2 (R&D Systems, Catalog # 11105-1) and EMCV-induced cytopathy was measured by crystal violet staining (orange line). The protective effect elicited by 1 ng/mL Recombinant Human IFN-alpha 2 was inhibited by treating the cells with increasing concentrations of the Type I IFN inhibitor, Recombinant Viral B18R (R&D Systems, Catalog # 8185-BR; blue line). The ED50 for this effect is typically 0.3–1.8 ng/mL.
Inhibition of EMCV-Induced Cytopathy by R&D Systems Recombinant Human IFN-beta and Neutralization by a Goat Anti-Human IFN-beta Polyclonal Antibody

IFN-beta-mediated Inhibition of EMCV-induced Cytopathy is Neutralized Using a Goat Anti-Human IFN-beta Polyclonal Antibody. The HeLa human cervical epithelial carcinoma cell line infected with encephalomyocarditis virus (EMCV) was treated with increasing concentrations of Recombinant Human IFN-beta (R&D Systems, Catalog # 8499-IF) and EMCV-induced cytopathy was measured by crystal violet staining (orange line). The ED50 for this effect is 5-30 pg/mL. The inhibitory effect elicited by 10 ng/mL Recombinant Human IFN-beta was neutralized by treating the cells with increasing concentrations of a Goat Anti-Human IFN-beta Antigen Affinity-purified Polyclonal Antibody (R&D Systems, Catalog # AF814; blue line). The ND50 is typically 0.05–0.2 µg/mL.
Type II Interferon

Type II Interferon Signaling Pathways
Type II Interferon
The type II interferon family consists of only one member, IFN-gamma, which functions as a noncovalently-linked homodimer. Although it is structurally unrelated to the type I interferons, IFN-gamma has similar anti-viral and immunomodulatory effects. Each monomer of IFN-gamma consists of six alpha helices, where two alpha helices from the first monomer are swapped with two alpha helices from the second monomer to form an intercalated dimer structure. IFN-gamma is primarily produced by CD8+ T cells, natural killer cells, and Th1 cells in response to a variety of stimuli, and can also be produced by antigen-presenting cells, including dendritic cells, macrophages, and B cells.
Type II IFN Receptor Complex
IFN-gamma signals through a heterodimeric receptor complex consisting of two subunits of IFN-gamma R1/CD119 and two subunits of IFN-gamma R2, which are expressed on most cell types. Both receptor subunits contain an extracellular cytokine receptor homology domain with two tandem, fibronectin type III domains. The IFN-gamma homodimer initially binds to two chains of the high affinity IFN-gamma R1/CD119 receptor subunit, which subsequently recruits two chains of the IFN-gamma R2 subunit to form an active receptor complex. In the absence of IFN-gamma R1/CD119, IFN-gamma R2 is incapable of binding to IFN-gamma, but its interaction with the IFN-gamma-IFN-gamma R1/CD119 complex not only strengthens the binding of IFN-gamma with IFN-gamma R1/CD119, but is also necessary for activating downstream signaling pathways.
Type II IFN Signaling
Similar to type I interferon signaling, activation of the IFN-gamma receptor promotes Jak-STAT signaling, leading to the STAT-induced expression of multiple target genes. STAT1 is the primary mediator of IFN-gamma signaling. Following its phosphorylation, it homodimerizes and translocates to the nucleus, where it binds to IFN-gamma-activated sequence (GAS) elements in the promoters of target genes to regulate their transcription. In addition to the Jak-STAT pathway, IFN-gamma can also activate the PI 3-K-Akt, NF-kappa B, and MAPK signaling pathways. Similar to type I interferons, IFN-gamma signaling induces the expression of a variety of pro-inflammatory mediators, cytokines, and chemokines, but it typically only induces a subset of the target genes that are induced by the type I IFN-alpha and IFN-beta proteins. IFN-gamma signaling also promotes both innate and adaptive immune responses by regulating the activities of a variety of immune cell types. IFN-gamma promotes activation and M1 polarization of macrophages, increases the expression of antigen processing and presentation molecules on antigen-presenting cells to promote T cell activation, drives Th1 cell development and proliferation, and enhances the cytotoxic activity of natural killer cells. As a result, IFN-gamma plays a central role in conferring protective immunity in infectious diseases and cancer.
Type II Interferon, Receptors, and Inhibitor - Products by Molecule
Inhibition of EMCV-Induced Cytopathy by R&D Systems Recombinant Human IFN-gamma and Neutralization by a Mouse Anti-Human IFN-gamma Monoclonal Antibody

IFN-gamma-mediated Inhibition of EMCV-induced Cytopathy is Neutralized Using a Mouse Anti-Human IFN-gamma Monoclonal Antibody. The HeLa human cervical epithelial carcinoma cell line infected with encephalomyocarditis virus (EMCV) was treated with increasing concentrations of Recombinant Human IFN-gamma (R&D Systems, Catalog # 285-IF) and EMCV-induced cytopathy was measured by crystal violet staining (orange line). The ED50 for this effect is 0.15-0.75 ng/mL. The inhibitory effect elicited by 1 ng/mL Recombinant Human IFN-gamma was neutralized by treating the cells with increasing concentrations of a Mouse Anti-Human IFN-gamma Monoclonal Antibody (R&D Systems, Catalog # MAB285; blue line). The ND50 is typically 0.02–0.6 µg/mL.
Measurement of Anti-NKp44-Induced IFN-gamma Secretion by Natural Killer Cells Using the Human IFN-gamma Quantikine™ ELISA Kit

Detection of Anti-NKp44-induced IFN-gamma Secretion by IL-2-activated Human Natural Killer Cells. Human peripheral blood natural killer (NK) cells were isolated using the MagCellect™ Human NK Cell Isolation Kit (R&D Systems, Catalog # MAGH109). Isolated cells were treated with Recombinant Human IL-2 (R&D Systems, Catalog # 202-IL) and the indicated concentrations of an immobilized Goat Anti-Human NKp44 Antigen Affinity-purified Polyclonal Antibody (R&D Systems, Catalog # AF2249). IFN-gamma secretion was measured using the Human IFN-gamma Quantikine ELISA Kit (R&D Systems, Catalog # DIF50C).
Detection of IFN-gamma in PMA/Monensin/Ca2+/Ionomycin-treated Mouse Splenocytes by Flow Cytometry

Detection of IFN-gamma in Mouse Splenocytes by Flow Cyotmetry. Mouse splenocytes were either (A) stimulated for 4 hours with Cell Activation Cocktail (Tocris, Catalog # 5476) or (B) unstimulated and then stained with an Alexa Fluor® 700-conjugated Rat Anti-Mouse IFN-gamma Monoclonal Antibody (R&D Systems, Catalog # IC485N) and a PE-conjugated Rat Anti-Mouse CD4 Monoclonal Antibody (R&D Systems, Catalog # FAB554P). Quadrant markers were set based on staining with an Alexa Fluor 700-conjugated Rat IgG2A Isotype Control Antibody (R&D Systems, Catalog # IC006N). To facilitate intracellular staining, cells were fixed with Flow Cytometry Fixation Buffer (R&D Systems, Catalog # FC004) and permeabilized with Flow Cytometry Permeabilization/Wash Buffer (R&D Systems, Catalog # FC005).
Protein Characterization Using SEC-MALS Analysis
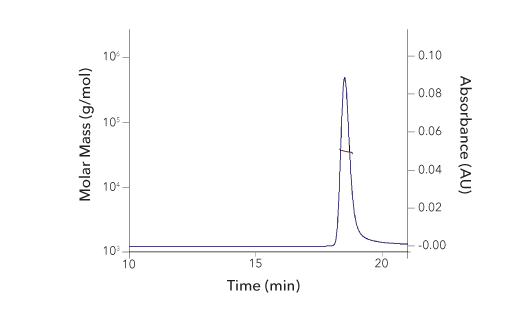
Recombinant Human IFN‑ gamma Protein SEC-MALS. Recombinant Human IFN-gamma (Catalog # 285-IF) has a molecular weight (MW) of 34.9 kDa as analyzed by SEC-MALS, suggesting that this protein is a homodimer. MW may differ from predicted MW due to post-translational modifications (PTMs) present (i.e. Glycosylation).
| SEC-MALS Data | Result |
| Retention Time | 18.3-18.8 min |
| MW-Predicted (Monomer) | 16.9 kDa |
| MW-MALS | 34.9 kDa |
| Polydispersity | 1.001 |
| System Suitability: BSA Monomer 66.4 ± 3.32 kDa | Pass |
Type III Interferons

Type III Interferon Signaling Pathways
Type III Interferons
The type III interferon family consists of IL-29/IFN-lambda 1, IL-28A/IFN-lambda 2, IL-28B/IFN-lambda 3, and IFN-lambda 4. Members of this family share a high degree of sequence homology, with IL-29/IFN-lambda 1 being 81% homologous to IL-28A/IFN-lambda 2 and IL-28B/IFN-lambda 3, and IL-28A and IL-28B sharing 96% amino acid identity. In mice, both IL-29/IFN-lambda 1 and IFN-lambda 4 have been characterized as pseudogenes. Human IFN-lambda 4 was also originally characterized as a pseudogene, but it has since been found that a dinucleotide frameshift variant generates a functional protein. Type III interferons are structurally similar to the IL-10 family cytokines, but their functional effects resemble those of the type I interferon family, resulting in their categorization as a new family of interferons. Similar to the type I interferons, expression of the type III interferons is induced in response to many different types of viruses and TLR agonists, and they have similar anti-viral and anti-proliferative effects. One notable difference in the expression of type I and type III interferons that has been reported is that type I interferons are induced and resolved rapidly following infection, while type III interferon expression is delayed and sustained, but the significance of this temporal regulation is not currently well understood. Type III interferons are primarily produced by dendritic cells, monocytes, mast cells, and epithelial cells.
Type III IFN Receptor Complex
An important distinction between type I and type III interferons is that they signal through different receptor complexes, and type III interferons primarily act on epithelial cells. While type I interferons utilize a receptor complex composed of IFN-alpha/beta RI and IFN-alpha/beta R2 that is broadly expressed on most cell types, type III interferons signal through a heterodimeric receptor complex consisting of the long IL-28 R alpha/IFN-lambda R1 transmembrane chain paired with the short IL-10 R beta 2/IL-10 R2 transmembrane chain. The IL-10 R beta 2/IL-10 R2 subunit is also part of the receptor complexes for IL-10, IL-22, and IL-26, and similar to these cytokines, type III interferons initially bind to the alpha chain subunit of their receptor, which subsequently recruits the IL-10 R beta 2/IL-10 R2 chain. Although IL-10 R beta 2/IL-10 R2 is constitutively expressed on most cell types, the expression of IL-28 R alpha/IFN-lambda R1 is more restricted to epithelial cells, subsets of myeloid cells, and neuronal cells. As a result, type III interferons primarily act on epithelial cells at mucosal barrier surfaces such as the respiratory tract, gastrointestinal tract, and the blood/brain barrier.
Type III IFN Signaling
Although type III interferons signal through a different receptor complex than type I interferons, both types of interferons activate similar downstream signaling pathways. The Jak-STAT pathway is the primary signaling pathway activated following formation of either the active type I or type III interferon receptor complexes. The Jak family kinases phosphorylate tyrosines in the intracellular domain of the interferon receptor, creating docking sites for STAT proteins, which are subsequently phosphorylated by the Jak family kinases. Phosphorylated STAT1 and STAT2 associate with IRF9 to form the IFN-stimulated gene factor 3 (ISGF3) complex, which then translocates to the nucleus where it binds to IFN-stimulated response elements (ISREs) located in the promoter regions of various IFN-stimulated genes to regulate their expression. In addition to Jak-STAT signaling, type III interferons can also activate other STAT proteins as well as MAPK signaling pathways. Together, these pathways regulate the expression of genes that direct the anti-viral and anti-proliferative effects of type III IFNs. In addition, type III interferons have immunomodulatory properties that affect the innate and adaptive immune responses following infection. Type III interferons have been shown to regulate the expression of specific cytokines and chemokines by dendritic cells, monocytes, and macrophages. In the case of dendritic cells, this can result in enhanced Th1 cell differentiation and inhibition of both Th2 and Th17 development. Type III interferons have also been shown to enhance T cell proliferation and the cytotoxic activity of CD8+ T cells, although the mechanisms by which they do so remain unclear as type III interferons are not thought to act directly on T cells.
Type III Interferons - Products by Molecule
Type III Interferon Receptors and Inhibitor - Products by Molecule
Inhibition of EMCV-Induced Cytopathy by R&D Systems Recombinant Human IL-29/IFN-lambda 1 and Neutralization by a Mouse Anti-Human IL-29/IFN-lambda 1 Antibody

IL-29/IFN-lambda 1-mediated Inhibition of EMCV-induced Cytopathy is Neutralized Using a Mouse Anti-Human IL-29/IFN-lambda 1 Monoclonal Antibody. The HepG2 human hepatocellular carcinoma cell line infected with encephalomyocarditis virus (EMCV) was treated with increasing concentrations of Recombinant Human IL-29/IFN-lambda 1 (R&D Systems, Catalog # 1598-IL) and anti-viral activity was measured by Resazurin (R&D Systems, Catalog # AR002; orange line). The ED50 for this effect is 1-5 ng/mL. The inhibitory effect elicited by 40 ng/ mL Recombinant Human IL-29/IFN-lambda 1 was neutralized by treating the cells with increasing concentrations of a Mouse Anti-Human IL-29/IFN-lambda 1 Monoclonal Antibody (R&D Systems, Catalog # MAB15981; blue line). The ND50 is typically 1–4 µg/mL.
Detection of IL-28 R alpha/IFN-lambda R1 in PMA/Ca2+/Ionomycin-treated PBMCs by Flow Cytometry

Detection of IL-28 R alpha/IFN-lambda R1 in Peripheral Blood Mononuclear Cells by Flow Cytometry. Peripheral blood mononuclear cells either (A) untreated or (B) treated with 50 ng/mL PMA and 200 ng/mL calcium ionomycin overnight were stained with a PE-conjugated Mouse Anti-Human IL-28 R alpha/IFN-lambda R1 Monoclonal Antibody (R&D Systems, Catalog # FAB5260P) and an APC-conjugated Mouse Anti-Human CD3 epsilon Monoclonal Antibody (R&D Systems, Catalog # FAB100A). Quadrant markers were set based on staining with a PE-conjugated Mouse IgG1 Isotype Control Antibody (R&D Systems, Catalog # IC002P).
Interferon Intracellular Signaling
Interferon Intracellular Signaling - Products by Molecule
Featured Products for Interferon Research

Recombinant IFNs
Recombinant IFNs
Interferons play a critical role in protecting the host against a wide variety of invading pathogens. To study the biological effects of these molecules in different experimental systems, Bio-Techne offers a wide selection of human and mouse type I, type II, and type III interferons. Stringent production and purification standards ensure that R&D Systems™ proteins will provide researchers with industry-leading bioactivity and lot-to-lot consistency.
Viral IFN Inhibitors
Viral IFN Inhibitors
Viral interferon inhibitors allow researchers to assess the importance of interferons in controlling specific viral infections and can be used for applications such as growing IFN-sensitive viruses in different cell lines and vaccine development. Our catalog includes several viral interferon inhibitors such as Recombinant Viral B8R, a potent inhibitor of IFN-gamma, Recombinant Viral B18R, an inhibitor of all type I interferons, and Recombinant Viral 136R, an inhibitor of both type I and type III interferons.

Blocking/Neutralizing Antibodies for IFNs and IFN Receptors
Blocking/Neutralizing Antibodies for IFNs and IFN Receptors
Blocking and neutralizing antibodies bind to their targets and either directly interfere with their functions or negatively regulate their downstream cellular effects. Bio-Techne offers a large selection of R&D Systems™ blocking or neutralizing antibodies against interferons and interferon receptors that have been validated to inhibit the activities of their target molecules in relevant biological assays.
Featured Resources Related to Interferon and Virus Research

Interferons and Anti-Viral Small Molecules Product Guide
Interferons and Anti-Viral Small Molecules Product Guide
Explore our interferons product guide to learn more about type I, type II, and type III interferons and the signaling pathways that they activate. This guide includes a complete listing of our interferon products and provides multiple data examples to demonstrate the testing our scientists have performed to ensure that our products will provide superior performance and lot-to-lot consistency.

Significant Events in the History of Virology Poster
Significant Events in the History of Virology Poster
From 1796 when Edward Jenner administered the first smallpox vaccine to the outbreak of SARS-CoV-2 in late December 2019, there have been many notable events in the history of virology. Learn more in this poster about how the field of virology has advanced throughout history, including novel findings that have increased our understanding of specific viruses, the development of critical vaccines, and recent outbreaks of emerging viruses.

Cytokine Signaling Pathways
Cytokine Signaling Pathways
Cytokines activate a diverse array of intracellular signaling pathways that can induce processes such as cell proliferation, differentiation, migration, and inflammation. Explore the signaling pathways that are activated by different cytokine families, the primary target cells that they affect, and the biological effects that they mediate using our interactive signaling pathways.