TNFR Superfamily Co-Stimulatory Receptors
TNFRSF Co-Stimulatory Receptors as Immune Checkpoint Targets
In contrast to blocking inhibitory immune checkpoint receptors to improve anti-tumor immune responses, researchers are also looking at using agonists of co-stimulatory immune checkpoint receptors. The majority of these co-stimulatory receptors are members of the tumor necrosis factor receptor superfamily (TNFRSF). They are primarily expressed on the surface of T cells, B cells, and/or natural killer (NK) cells and upon activation, with either their ligands or agonistic antibodies, they promote signaling pathways that enhance the survival, proliferation, and effector functions of these cells. The anti-tumor effects of stimulating these receptors alone, or in combination with antibodies that block inhibitory receptors like CTLA-4 or PD-1, are being actively explored. Details about the primary TNFRSF co-stimulatory molecules that are currently being investigated as immuno-oncology targets are described in the sections below.
Bio-Techne offers R&D Systems™ bioactive recombinant proteins for all of the co-stimulatory TNF receptor superfamily members that are being investigated as immuno-oncology targets, and their ligands. Our portfolio includes proteins for a variety of different species with different tags including an expanding selection of Avi-tag biotinylated proteins. Additionally, we offer a wide selection of antibodies against these targets that are validated for a range of applications to help advance our understanding of the immune-stimulatory functions of these molecules.
Activation of 4-1BB by 4-1BB L Delivers a CD28-Independent Co-Stimulatory Signal
4-1BB, also known as CD137 and TNFRSF9, is widely expressed on a variety of immune cell types, including activated CD4+ and CD8+ T cells, regulatory T cells, natural killer cells, NKT cells, and dendritic cells.1-5 4-1BB binds with high affinity to its ligand, 4-1BB Ligand/TNFSF9, which is expressed on activated macrophages, B cells, and dendritic cells.6-8 This interaction transduces a co-stimulatory signal that promotes the activation, proliferation, and survival of CD4+ and CD8+T cells, independent of CD28 co-stimulatory signaling.8-11 Stimulation of 4-1BB on CD8+ T cells increases TCR signaling and the cytotoxic activity of these cells.12 Likewise, stimulation of 4-1BB on natural killer cells increases both the production of IFN-gamma and their cytolytic activity.13 4-1BB is up-regulated on human natural killer cells following Fc receptor ligation and subsequent 4-1BB stimulation enhances natural killer cell-mediated killing by antibody-dependent cell-mediated cytotoxicity.14-16 On dendritic cells (DCs), 4-1BB is strongly expressed during DC maturation and its stimulation promotes up-regulation of B7-1/CD80 and B7-2/CD86, increased cytokine production, and increased survival.3, 17
In mouse tumor models, researchers have observed potent anti-tumor immune responses following treatment with agonistic anti-4-1BB antibodies, which were attributed to an increase in CD8+ T cell activity and natural killer cell activity, combined with a decrease in the suppressive activity of regulatory T cells.18-20 Furthermore, studies utilizing anti-CTLA-4 antibodies with agonistic anti-4-1BB antibodies in mouse tumor models demonstrated that this combination therapy could synergistically improve anti-tumor immune responses.21-23 As a result, the effectiveness of agonistic anti-4-1BB antibodies alone, or in combination with either an agonistic anti-OX40 antibody, or with antagonistic antibodies against negative regulatory immune checkpoint targets, are currently being investigated for treating several different types of human cancers.24, 25
Ligation of CD27 by CD70 Promotes T Cell Expansion and Improves Anti-Tumor Immunity
CD27, also known as TNFRSF7, is primarily expressed on T cells, B cells, and natural killer cells. While CD27 is only weakly expressed on naïve CD4+ and CD8+ T cells, it is up-regulated following T cell activation.1, 2 CD27 binds to CD27 Ligand/CD70, expressed on activated dendritic cells, B cells, T cells, and natural killer cells, and this interaction provides a CD28 complementary T cell co-stimulatory signal.3-9 Ligation of T cell-expressed CD27 promotes antigen-specific CD4+ and CD8+ clonal expansion, supports Th1 and CD8+ effector T cell differentiation, and promotes effector T cell survival.5, 10-15 A soluble form of CD27 can also be produced by proteolytic shedding of CD27 from the surface of activated T cells.4 Although CD27 is not expressed on naïve B cells, it is up-regulated on activated B cells, and promotes the generation of effector and memory B cells, and B cell expansion in germinal centers.3, 4 Additionally, CD27 is expressed on natural killer cells and has been shown to enhance natural killer cell proliferation and IFN-gamma production following ligation.2
In mice bearing EL-4 lymphoma tumors, researchers demonstrated that transgenic expression of CD70 promotes tumor regression in a CD8+ T cell-, IFN-gamma-dependent manner.16 A mouse agonistic anti-CD27 antibody was also shown to have anti-tumor effects in immunocompetent mice bearing syngeneic T cell or BCL1 lymphoma, and similar results were obtained in a mouse model of melanoma.17-19 A fully human agonistic anti-CD27 antibody was also shown to have potent anti-tumor efficacy in human CD27 transgenic syngeneic mouse tumor models.20 When combined with anti-CTLA-4 blocking antibodies, this human anti-CD27 antibody synergistically improved the anti-tumor immune response.21 As a result, agonistic anti-CD27 and anti-CD70 antibodies are being investigated in clinical trials for treating both hematologic malignancies and solid tumors.22, 23
CD40 Activation by CD40 L Indirectly Promotes T Cell Priming, Activation, and Th1 Polarization
CD40, also known as TNFRSF5, is a type I transmembrane receptor belonging to the TNF receptor superfamily that is expressed on the surface of antigen-presenting cells (APCs), including dendritic cells, macrophages, monocytes, and B cells.1,2 Its ligand, CD40 Ligand, is expressed on activated T cells and B cells, and its expression can be induced on natural killer cells, monocytes, basophils and mast cells in response to inflammation.3 Ligation of CD40 by CD40 Ligand promotes B cell activation, proliferation, and T cell-dependent humoral responses.3,4 Additionally, CD40 activation triggers signaling pathways that lead to the production of cytokines, co-stimulatory molecules, adhesion molecules, and enzymes that affect both cellular and humoral immuneresponses.5,6 Due to the ability of the CD40/CD40 Ligand interaction to affect the expression of co-stimulatory molecules on APCs and their cytokine production, including TNF-alpha and IL-12, CD40 signaling indirectly regulates T cell priming, T cell activation, and Th1 polarization.
CD40 has been found to be expressed on a wide range of tumors and has been shown to have context-dependent tumor-promoting or tumor-inhibiting effects.6 While co-expression of CD40 and CD40 Ligand on tumor cells can promote their proliferation and motility, the CD40/CD40 Ligand interaction can also have direct cytotoxic effects on tumor cells or inhibit tumor growth through the activation of APCs. Significantly, agonistic anti-CD40 antibodies have been shown to stimulate potent anti-tumor CD8+ T cell responses in a syngeneic mouse lymphoma model.7-9 In patients with different types of advanced stage cancers, encouraging anti-tumor results have also been observed in phase I testing of both recombinant human CD40 Ligand and agonistic anti-CD40 antibodies.10-12 Many of these agonists are still being evaluated in ongoing clinical trials, suggesting that stimulating the CD40/CD40 Ligand pathway may be a promising future therapeutic strategy for treating some forms of cancer.13, 14
GITR Binding by GITR L Promotes T Cell Activation and Inhibits Regulatory T Cell Activity
Glucocorticoid-induced TNF receptor family-related protein (GITR), also known as TNFRSF18, is a co stimulatory type I transmembrane receptor belonging to the TNF receptor superfamily. It is expressed at low levels on resting CD4+ and CD8+ T cells and is up-regulated following T cell activation.1-4 GITR is also expressed on natural killer cells and is expressed at high levels on CD4+CD25+ regulatory T cells, where it is further up-regulated upon activation.5-7 GITR binds to a type II transmembrane protein known as GITR Ligand/TNFSF18. GITR Ligand is primarily expressed on antigen-presenting cells, including dendritic cells (DCs), macrophages, and B cells, and has been shown to be up-regulated on DCs following activation.8-10 Ligation of GITR by GITR Ligand stimulates anti-CD3-induced T cell proliferation, co-stimulates CD4+ and CD8+ T cell activation, and promotes NK cell activation, cytotoxicity, and IFN-gamma production.1, 3, 4, 7, 8 Additionally, stimulation of GITR on regulatory T cells, promotes cell proliferation, but inhibits their suppressive activity.4, 6, 11
GITR is considered to be an intriguing target for cancer immunotherapy as GITR agonists activate CD8+ and CD4+ effector T cells, while also inhibiting the function of regulatory T cells. In several syngeneic mouse tumor models, both a recombinant mouse GITR Ligand-Fc fusion protein and agonistic anti-GITR antibodies were found to stimulate potent anti-tumor immune responses.12-16 The effectiveness of these agonists was attributed to their ability to promote increased tumor infiltration by CD4+ and CD8+ T cells, expansion of IFN-gamma-secreting CD8+ T cells and NK cells, and depletion of intratumoral regulatory T cells.12-16 Furthermore, combination therapies using anti-CTLA-4 antibodies and anti-GITR antibodies displayed synergistic anti-tumor activity.12, 17 As a result, a number of agonistic anti-GITR antibodies are being evaluated in phase I clinical trials.18, 19
OX40 Signaling Stimulates T Cell Activation, Inhibits Treg Activity, and Promotes Tumor Regression
OX40, also known as TNFRSF4 and CD134, is a type I transmembrane protein that is up-regulated and transiently expressed on activated CD4+ and CD8+ T cells, activated natural killer cells, NKT cells, and neutrophils.1-4 It binds to a type II transmembrane protein known as OX40 Ligand (OX40 L), which is inducibly expressed on activated antigen-presenting cells, including B cells, macrophages, and dendritic cells (DCs), in response to inflammatory cytokines or following activation of the B cell receptor (BCR), CD40, or toll-like receptors (TLRs).3, 5 The interaction between OX40 and OX40 L, provides a potent T cell co-stimulatory signal that promotes T cell proliferation, survival, and effector functions.3-5 Additionally, OX40 signaling can inhibit the suppressive activity of regulatory T cells and stimulate natural killer cell cytotoxicity and IFN-gamma production, in the presence of antibody:Fc receptor binding.2, 6, 7
Both agonistic anti-OX40 monoclonal antibodies and an OX40 Ligand-Fc fusion protein were shown to promote tumor regression in mouse tumor models, which was attributed to increased T cell infiltration of the tumor, and the expansion and effector functions of both CD4+ and CD8+ T cells.8-12 Several studies in preclinical models also demonstrated that combined treatment using either anti-CTLA-4 or anti-PD-1 blocking antibodies with an agonistic anti-OX40 antibody could synergistically improve the anti-tumor immune response.13-15 A mouse anti-OX40 agonistic antibody was also tested in patients with advanced cancers and was shown to be well-tolerated and to have similar immuno-stimulatory effects as were previously observed in mouse tumor models.16 Collectively, the data suggest that OX40 agonists alone, or in combination with inhibitory immune checkpoint blockade, may represent potential strategies for treating some forms of cancer.
TNFRSF Co-Stimulatory Immune Checkpoint Receptors & Ligands - Products by Molecule
TNF Receptor Superfamily Members Can Promote T Cell and Natural Killer Cell Activation
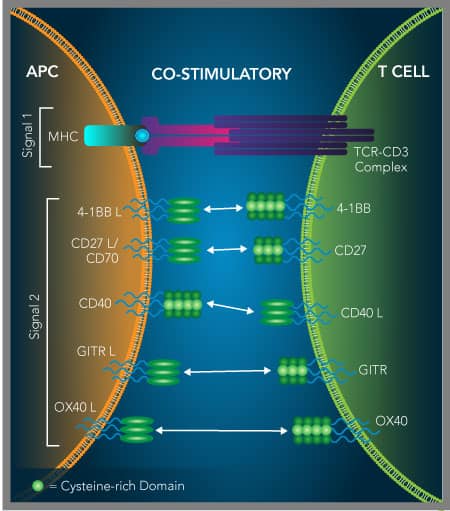
Activation of immune cell co-stimulatory receptors belonging to the TNF receptor superfamily is another strategy being investigated for cancer immunotherapy. As an alternative to blocking inhibitory immune checkpoint receptors to restore antitumor immune responses, agonists of co-stimulatory immune checkpoint receptors are also being explored. Many of these co-stimulatory receptors belong to the TNF receptor superfamily and have been shown to be involved in enhancing the proliferation and effector functions of T cells and/or natural killer (NK) cells, while some also inhibit the activity of regulatory T cells.
Analysis of the Binding of R&D Systems Recombinant Human 4-1BB and 4-1BB L Proteins
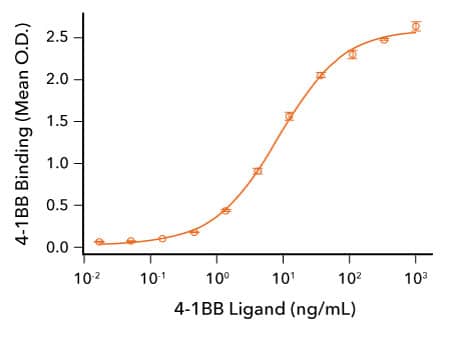
Human 4-1BB Ligand Binds 4-1BB. Recombinant Human 4-1BB/TNFRSF9 (R&D Systems, Catalog # 9220-4B) was immobilized at 50 ng/mL and the indicated concentrations of Recombinant Human 4-1BB Ligand (R&D Systems, Catalog # 2295-4L) were added. Recombinant Human 4-1BB Ligand bound with an ED50 of 2.5-15 ng/mL.
Protein Characterization Using SEC-MALS Analysis
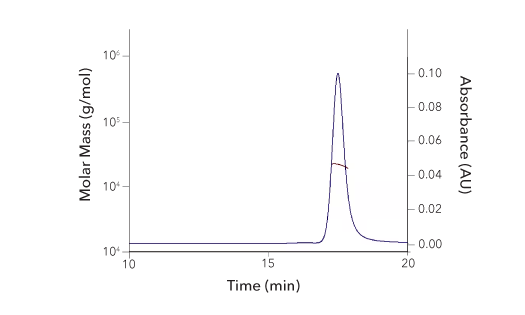
Recombinant Human 4‑1BB/TNFRSF9/CD137 Protein SEC-MALS. Recombinant Human 4-1BB/TNFRSF9 (Catalog # 9220-4B) has a molecular weight (MW) of 21.8 kDa as analyzed by SEC-MALS, suggesting that this protein is a monomer. MW may differ from predicted MW due to post-translational modifications (PTMs) present (i.e. Glycosylation).
| SEC-MALS Data | Result |
| Retention Time | 17.3-17.9 min |
| MW-Predicted (Monomer) | 18.0 kDa |
| MW-MALS | 21.8 kDa |
| Polydispersity | 1.002 |
| System Suitability: BSA Monomer 66.4 ± 3.32 kDa | Pass |
Assessment of the Bioactivity of R&D Systems Recombinant Human CD27 Ligand Protein

CD27 Ligand/CD70 Induces IL-8 Secretion by Human Fibrosarcoma Cells. HT1080 human fibrosarcoma cells transfected with human CD27 were treated with the indicated concentrations of Recombinant Human CD27 Ligand/TNFSF7 (R&D Systems, Catalog # 9328-CL). IL-8 secretion was measured in cell culture supernatants using the Human IL-8/CXCL8 QuantikineTM ELISA Kit (R&D Systems, Catalog # D8000C). The ED50 for this effect is 5-25 ng/mL.
Analysis of the Binding of R&D System Recombinant Human CD40 and CD40 L Proteins

CD40/TNFRSF5 Binds to CD40 Ligand. Recombinant Human CD40 Ligand/TNFSF5 (R&D Systems, Catalog # 6420-CL; HEK293-expressed) was immobilized at 2 μg/mL, 100 μL/well and the indicated concentrations of Avi-tag Biotinylated Recombinant Human CD40/TNFRSF5 Fc Chimera (R&D Systems, Catalog # AVI10380) were added. The concentration of Avi-tag Biotinylated Recombinant Human CD40 that produces 50% optimal binding is 40-320 ng/mL.
Analysis of the Binding of R&D Systems Recombinant Human GITR and GITR L Proteins

GITR Binds to GITR Ligand. Recombinant Human GITR Ligand/TNFSF18 (R&D Systems, Catalog # 6987-GL) was immobilized at 0.5 μg/mL, 100 μL/well and the indicated concentrations of Avi-tag Biotinylated Recombinant Human GITR/TNFRSF18 Fc Chimera (R&D Systems, Catalog # AVI689) were added. Avi-tag Biotinylated Recombinant Human GITR/TNFRSF18 bound with an ED50 of 0.05-0.5 μg/mL.
Assessment of the Binding Activity and Purity of R&D Systems Recombinant Human OX40 Protein

OX40 Ligand Binds to OX40/TNFRSF4. Recombinant Human OX40/TNFRSF4 (R&D Systems, Catalog # 9969-OX) was immobilized at 0.25 μg/mL and the indicated concentrations of Recombinant Human OX40 Ligand/TNFSF4 (R&D Systems, Catalog # 1054-OX) were added. Recombinant Human OX40 Ligand bound with an ED50 of 0.25-1.5 ng/mL.

Assessment of the Purity of Recombinant Human OX40/TNFRSF4 by SDS-PAGE. The purity of Recombinant Human OX40/TNFRSF4 (R&D Systems, Catalog # 9969-OX) was assessed by SDS-PAGE analysis under reducing (R) and nonreducing (NR) conditions and visualized by Coomassie® Blue staining.
4-1BB - 4-1BB L
1. Wen, T.et al. (2002) 4-1BB ligand-mediated costimulation of human T cells induces CD4 and CD8 T cell expansion, cytokine production, and the development of cytolytic effector function. J. Immunol. 168:4897. PMID: 11994439
2. Zheng, G. et al (2004) The 4-1BB costimulation augments the proliferation of CD4+CD25+ regulatory T cells. J. Immunol. 173:2428. PMID: 15294956
3. Choi, B.K. et al (2009) 4-1BB functions as a survival factor in dendritic cells. J. Immunol. 182:4107. PMID: 19299708
4. Vinay, D.S. & B.S. Kwon:(2011) 4-1BB signaling beyond T cells. Cell. Mol. Immunol. 8:281. PMID: 21217771
5. Kim, D. et al (2008) 4-1BB engagement costimulates NKT cell activation and exacerbates NKT cell ligand-induced airway hyperresponsiveness and inflammation. J. Immunol. 180:2062. PMID: 18250411
6. Goodwin, R.G. et al (1993) Molecular cloning of a ligand for the inducible T cell gene 4-1BB: a member of an emerging family of cytokines with homology to tumor necrosis factor. Eur. J. Immunol. 23:2631. PMID: 8405064
7. Pollok, K.E. et al (1994) 4-1BB T-cell antigen binds to mature B cells and macrophages, and costimulates anti-mu-primed splenic B cells. Eur. J. Immunol. 24:367. PMID: 8299685
8. DeBenedette, M.A. et al (1997) Costimulation of CD28- T lymphocytes by 4-1BB ligand. J. Immunol. 158:551. PMID: 8992967
9. Chu, N.R. et al (1997) Role of IL-12 and 4-1BB ligand in cytokine production by CD28+ and CD28- T cells. J. Immunol. 158:3081. PMID: 9120260
10. Saoulli, K.S. et al (1998) CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J. Exp. Med. 187:1849. PMID: 9607925
11. Cannons, J.L. et al (2001) 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J. Immunol. 167:1313. PMID: 11466348
12. Shuford, W.W. et al (1997) 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J. Exp. Med. 186:47. PMID: 9206996
13. Melero, I. et al (1998) NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-41BB monoclonal antibodies. Cell. Immunol. 190:167. PMID: 9878117
14. Lin, W. et al (2008) Fc-dependent expression of CD137 on human NK cells: insights into "agonistic" effects of anti-CD137 monoclonal antibodies. Blood 112:699. PMID: 18519814
15. Kohrt, H.E. et al (2011) CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood 117:2423. PMID: 21193697
16. Wang, W. et al (2015) NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front. Immunol. 6:368. PMID: 26284063
17. Kuang, Y. et al (2012) Effects of 4-1BB signaling on the biological function of murine dendritic cells. Oncol. Lett. 3:477. PMID: 22740935
18. Melero, I. et al (1997) Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat. Med. 3:682. PMID: 9176498
19. Xu, D. et al (2004) NK and CD8+ T cell-mediated eradication of poorly immunogenic B16-F10 melanoma by the combined action of IL-12 gene therapy and 4-1BB costimulation. Int. J. Cancer 109:499. PMID: 14991570
20. Buchan, S.L.et al. (2018) Antibodies to costimulatory receptor 4-1BB enhance anti-tumor immunity via T regulatory cell depletion and promotion of CD8 T cell effector function. Immunity 49:958. PMID: 30446386
21. Kocak, E. et al (2006) Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res. 66:7276. PMID: 16849577
22. Li, B. et al (2007) Established B16 tumors are rejected following treatment with GM-CSF-secreting tumor cell immunotherapy in combination with anti-4-1BB mAb. Clin. Immunol. 125:76. PMID: 17706463
23. Curran, M. et al (2011) Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS One 6:19499. PMID: 21559358
24. Dempke, W.C.M. et al (2017) Second- and third-generation drugs for immuno-oncology treatment-the more the better? Eur. J. Cancer 74:55. PMID: 28335888
25. Marin-Acevedo, J.A.:(2018) Next generation of immune checkpoint therapy in cancer: new developments and challenges. J. Hematol. Oncol. 11:39. PMID: 29544515
CD27- CD27 L/CD70
1. Loenen, W.A.et al. (1992) The CD27 membrane receptor, a lymphocyte-specific member of the nerve growth factor receptor family, gives rise to a soluble form by protein processing that does not involve receptor endocytosis. Eur. J. Immunol. 22:447. PMID: 1311261
2. Takeda, K. et al (2000) CD27-mediated activation of murine NK cells. J. Immunol. 164:1741. PMID: 10657619
3. Lens, S.M. et al (1998) Control of lymphocyte function through CD27-CD70 interactions. Semin. Immunol. 19:491. PMID: 9826582
4. Borst, J. et al (2005) CD27 and CD70 in T cell and B cell activation. Curr. Opin. Immunol. 17:275. PMID: 15886117
5. Goodwin, R.G. et al (1993) Molecular and biological characterization of a ligand for CD27 defines a new family of cytokines with homology to tumor necrosis factor. Cell 73:447. PMID: 8387892
6. Bowman, M.R. et al (1994) The cloning of CD70 and its identification as the ligand for CD27. J. Immunol. 152:1756. PMID: 8120384
7. Hintzen, R.Q. et al (1994) CD70 represents the human ligand for CD27. Int. Immunol. 6:477. PMID: 8186199
8. Tesselaar, K. et al (2003) Expression of the murine CD27 ligand CD70 in vitro and in vivo. J. Immunol. 170:33. PMID: 12496380
9. Nolte, M.A. et al (2009) Timing and tuning of CD27-CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol. Rev. 229:216. PMID: 19426224
10. Van Oosterwijk, M.F. et al (2007) CD27-CD70 interactions sensitise naive CD4+ T cells for IL-12-induced Th1 cell development. Int. Immunol. 19:713. PMID: 17548342
11. Xiao, Y. et al (2008) CD27 instructs CD4+ T cells to provide help for the memory CD8+ T cell response after protein immunization. J. Immunol. 181:1071. PMID: 18606659
12. Rowley, T.F. & A.A. Shamkhani:(2004) Stimulation by soluble CD70 promotes strong primary and secondary CD8+ cytotoxic T cell responses in vivo. J. Immunol. 172:6039. PMID: 15128787
13. Van Gisbergen, K. et al (2011) The costimulatory molecule CD27 maintains clonally diverse CD8(+) T cell responses of low antigen affinity to protect against viral variants. Immunity 35:97. PMID: 21763160
14. Peperzak, V. et al (2010) The Pim kinase pathway contributes to survival signaling in primed CD8+ T cells upon CD27 costimulation. J. Immunol. 185:6670. PMID: 21048108
15. Pen, J.J. et al (2013) Modulation of regulatory T cell function by monocyte-derived dendritic cells matured through electroporation with mRNA encoding CD40 ligand, constitutively active TLR4, and CD70. J. Immunol. 191:1976. PMID: 23842750
16. Arens, R. et al (2004) Tumor rejection induced by CD70-mediated quantitative and qualitative effects on effector CD8+ T cell formation. J. Exp. Med. 199:1595. PMID: 15184507
17. Sakanishi, T. & H. Yagita:(2010) Anti-tumor effects of depleting and non-depleting anti-CD27 monoclonal antibodies in immune-competent mice. Biochem. Biophys. Res. Commun. 393:829. PMID: 20171165
18. French, R.R. et al (2007) Eradication of lymphoma by CD8 T cells following anti-CD40 monoclonal antibody therapy is critically dependent on CD27 costimulation. Blood 109:4810. PMID: 17311995
19. Roberts, D.J. et al (2010) Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8(+) T cells. J. Immunother. 33:769. PMID: 20842060
20. He, L-Z. et al (2013) Agonist anti-human CD27 monoclonal antibody induces T cell activation and tumor immunity in human CD27-transgenic mice. J. Immunol. 191:4174. PMID: 24026078
21. He, L-Z. et al (2013) Combination therapies augment the anti-tumor activity of agonist CD27 mAb in human CD27 transgenic mouse models. J. Immunother. Cancer 1:76. PMID:
22. Dempke, W.C.M. et al (2017) Second- and third-generation drugs for immuno-oncology treatment-the more the better? Eur. J. Cancer 74:55. PMID: 28335888
23. Marin-Acevedo, J.A.:(2018) Next generation of immune checkpoint therapy in cancer: new developments and challenges. J. Hematol. Oncol. 11:39. PMID: 29544515
CD40 - CD40 L
1. van Kooten, C. & J. Banchereau:(1997) Functions of CD40 on B cells, dendritic cells and other cells. Curr. Opin. Immunol. 9:330. PMID: 9203418
2. Schonbeck, U. et al (1997) Ligation of CD40 activates interleukin 1beta-converting enzyme (caspase-1) activity in vascular smooth muscle and endothelial cells and promotes elaboration of active interleukin 1beta. J. Biol. Chem. 272:19569. PMID: 9235962
3. Elgueta, R. et al (2009) Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 229:152. PMID: 19426221
4. Rickert, R.C. et al (2011) Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol. Rev. 244:115. PMID: 22017435
5. Schonbeck, U. et al (2001) The CD40/CD154 receptor/ligand dyad. Cell. Mol. Life Sci. 58:4. PMID: 11229815
6. Kawabe, T. et al (2011) CD40/CD40 ligand interactions in immune responses and pulmonary immunity. Nagoya J. Med. Sci. 73:69. PMID: 21928689
7. van Kooten, C. & J. Banchereau:(2000) CD40-CD40 ligand. J. Leukoc. Biol. 67:2. PMID: 10647992
8. French, R.R. et al (1999) CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat. Med. 5:548. PMID: 10229232
9. Tutt, A.L. et al (2002) T cell immunity to lymphoma following treatment with anti-CD40 monoclonal antibody. J. Immunol. 168:2720. PMID: 11884438
10. Vonderheide, R.H. et al (2001) Phase I study of recombinant human CD40 ligand in cancer patients. J. Clin. Oncol. 19:3280. PMID: 11432896
11. Vonderheide, R.H. et al (2007) Prospect of targeting the CD40 pathway for cancer therapy. Clin. Cancer Res. 13:1083. PMID: 17317815
12. Vonderheide, R.H. et al (2013) Agonistic CD40 antibodies and cancer therapy. Clin. Cancer Res. 19:1035. PMID: 23460534
13. Dempke, W.C.M. et al (2017) Second- and third-generation drugs for immuno-oncology treatment-the more the better? Eur. J. Cancer 74:55. PMID: 28335888
14. Marin-Acevedo, J.A.:(2018) Next generation of immune checkpoint therapy in cancer: new developments and challenges. J. Hematol. Oncol. 11:39. PMID: 29544515
GITR - GITR L
1. Nocentini, G.et al. (1997) A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc. Natl. Acad. Sci. USA 94:6216. PMID: 9177197
2. Kwon, B. et al (1999) Identification of a novel activation-inducible protein of the tumor necrosis factor receptor superfamily and its ligand. J. Biol. Chem. 274:6056. PMID: 10037686
3. Gurney, A.L. et al (1999) Identification of a new member of the tumor necrosis factor family and its receptor, a human ortholog of mouse GITR. Curr. Biol. 9:215. PMID: 10074428
4. Ronchetti, S. et al (2004) GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur. J. Immunol. 34:613. PMID: 14991590
5. Shimizu, J. et al (2002) Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 3:135. PMID: 11812990
6. Kanamaru, F. et al (2004) Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J. Immunol. 172:7306. PMID: 15187106
7. Hanabuchi, S. et al (2006) Human plasmacytoid predendritic cells activate NK cells through glucocorticoid-induced tumor necrosis factor receptor-ligand (GITRL). Blood 107:3617. PMID: 16397134
8. Tone, M. et al (2003) Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc. Natl. Acad. Sci, USA 100:15059. PMID: 14608036
9. Kim, J.D. et al (2003) Cloning and characterization of GITR ligand. Genes Immunol. 4:564. PMID: 14647196
10. Yu, K. et al (2003) Identification of a ligand for glucocorticoid-induced tumor necrosis factor receptor constitutively expressed in dendritic cells. Biochem. Biophys. Res. Commun. 310:433. PMID: 14521928
11. McHugh, R.S. et al (2002) CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity 16:311. PMID: 11869690
12. Hu,, P. et al (2008) Construction and preclinical characterization of Fc-mGITRL for the immunotherapy of cancer. Clin. Cancer Res. 14:579. PMID: 18223234
13. Ko, K. et al (2005) Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating FoxP3+CD25+CD4+ regulatory T cells. J. Exp. Med. 202:885. PMID: 16186187
14. Zhou, P. et al (2007) Pivotal roles of CD4+ effector T cells in mediating agonistic anti-GITR mAb-induced immune activation and tumor immunity in CT26 tumors. J. Immunol. 179:7365. PMID: 18025180
15. Coe, D. et al (2010) Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy. Cancer Immunol. Immunother. 59:1367. PMID: 20480365
16. Cohen, A.D. et al (2010) Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS One 5:10436. PMID: 20454651
17. Mitsui, J. et al (2010) Two distinct mechanisms of augmented antitumor activity by modulation of immunostimulatory/inhibitory signals. Clin. Cancer Res. 16:2781. PMID: 20460483
18. Dempke, W.C.M. et al (2017) Second- and third-generation drugs for immuno-oncology treatment-the more the better? Eur. J. Cancer 74:55. PMID: 28335888
19. Marin-Acevedo, J.A. et al (2018) Next generation of immune checkpoint therapy in cancer: new developments and challenges. J. Hematol. Oncol. 11:39. PMID: 29544515
OX40 - OX40 L
1. Gramaglia, I. et al. (1998) OX-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J. Immunol. 161:6510. PMID: 9862675
2. Turaj, A.H. et al (2018) Augmentation of CD134 (OX40)-dependent NK anti-tumour activity is dependent on antibody cross-linking. Sci. Rep. 8:2278. PMID: 29396470
3. Croft, M.:(2010) Control of immunity by the TNFR-related molecule OX40 (CD134). Ann. Rev. Immunol. 28:57. PMID: 20307208
4. Willoughby, J. et al (2017) OX40: Structure and function - What questions remain? Mol. Immunol. 83:13. PMID: 28092803
5. Mendel, I. & E.M. Shevach:(2005) Activated T cells express the OX40 ligand: requirements for induction and costimulatory function. Immunology 117:196. PMID: 16423055
6. Takeda, I. et al (2004) Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J. Immunol. 172:3580. PMID: 15004159
7. Aspeslagh, S. et al (2016) Rationale for anti-OX40 cancer immunotherapy. Eur. J. Immunol. 52:50. PMID: 26645943
8. Weinberg, A.D. et al (2000) Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J. Immunol. 164:2160. PMID: 10657670
9. Kjaergaard, J. et al (2000) Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 60:5514. PMID: 11034096
10. Ali, S.A. et al (2004) Anti-tumor therapeutic efficacy of OX40L in murine tumour model. Vaccine 22:3585. PMID: 15315837
11. Gough, M.J. et al (2008) OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 68:5206. PMID: 18593921
12. Piconese, S. et al (2008) OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J. Exp. Med. 205:825. PMID: 18362171
13. Redmond, W.L. et al (2014) Combined targeting of costimulatory (OX40) and coinhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust antitumor immunity. Cancer Immunol. Res. 2:142. PMID: 24778278
14. Marabelle, A. et al (2013) Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J. Clin. Invest. 123:2447. PMID: 23728179
15. Guo, Z. et al (2014) PD-1 blockade and OX40 triggering synergistically protects against tumor growth in a murine model of ovarian cancer. PLoS One 9:89350. PMID: 24586709
16. Curti, B.D. et al (2013) OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 73:7189. PMID: 24177180