Caspase Cleavage and Activation in Apoptosis
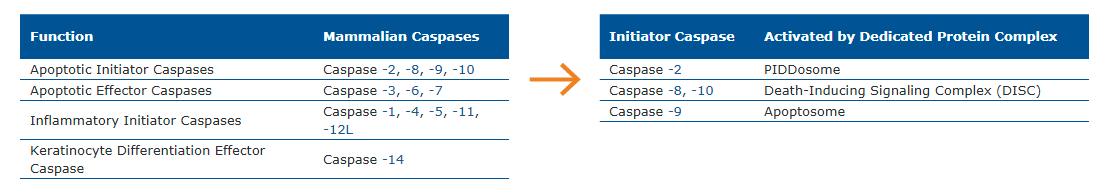
Caspases are a family of cysteine proteases found in the cytosol that act as the primary mediators of apoptosis. Based on their role in apoptosis or inflammation, caspases are subdivided into initiator and effector (executioner) groups. All caspases are synthesized as inactive zymogens containing a variable length pro-domain followed by a large and small subunit. Cleavage of caspases occurs at specific asparagine (Asn) residues located after the pro-domain and in between the large and small subunits, and leads to the formation of active heterotetramers.
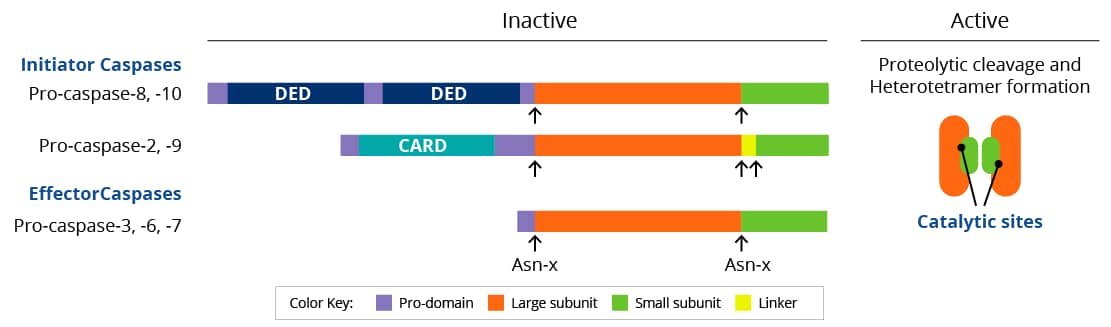
Domains: DED: Death-effector domain; CARD: Caspase activation and recruitment domains
Intrinsic or extrinsic death stimuli trigger the autocatalytic activation of apoptotic initiator caspases upon binding to dedicated protein complexes. Once activated, initiator caspases cleave pro-forms of executioner caspases to activate them, which in turn are responsible for the proteolytic processing of various cellular proteins.
Explore our the role of caspases in apoptosis with our Caspase Inhibitors.