AAV Characterization Methods
Characterization of adeno-associated virus (AAV) vectors is essential for developing effective, safe, consistent, and reproducible therapies.
While the traditional techniques of ELISAs and PCR serve their purpose during early-stage development, they may prove less efficient when scaling up AAV manufacturing.
As you move closer to the clinic, embracing automated platforms equipped with multi-attribute analysis capabilities not only accelerates processing times but also mitigates user errors. These tools play a pivotal role in monitoring Critical Quality Attributes (CQAs), thereby ensuring a safe, high-quality, consistent, and efficacious final product.
Table of Contents
Explore AAV characterization solutions by CQA below or get in touch with us to discuss your AAV characterization requirements.
AAV Titer Determination
Fast and reproducible AAV titer measurement is essential for ensuring safety and efficacy of your gene therapy product.
In partnership with PROGEN, we have automated the AAV titration ELISA, delivering rapid and efficient viral quantitation with superior specificity. These assays eliminate the need for time-consuming manual steps, such as standard curve preparation, so you can easily monitor AAV physical titer throughout development.
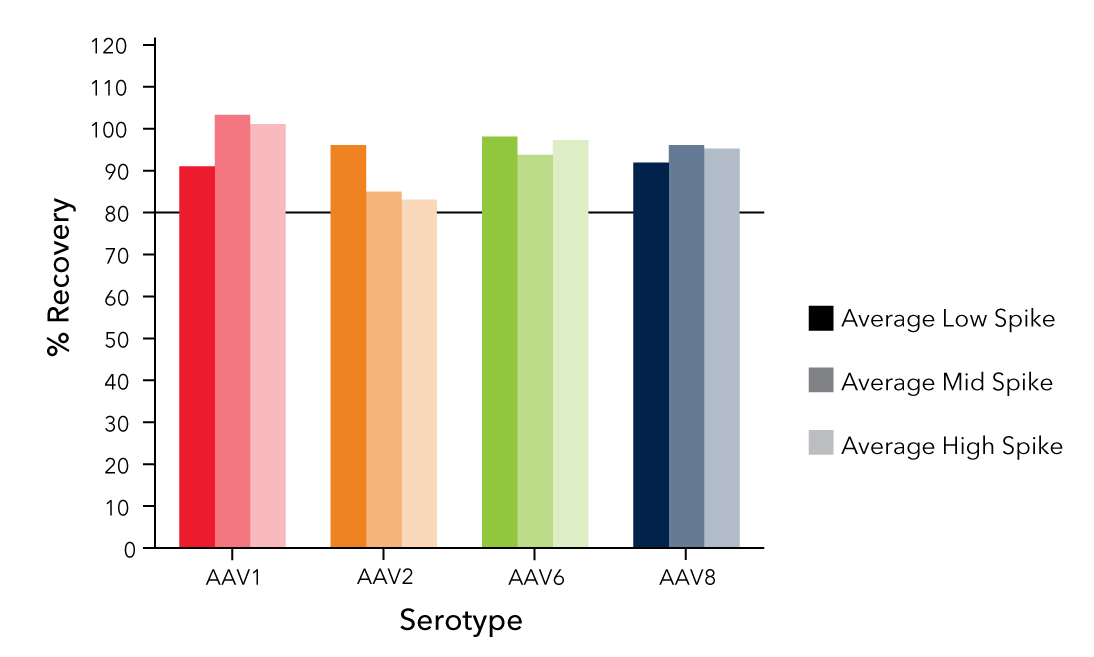

Recommended for AAV Titer Determination: Simple Plex™
Automated Simple Plex assays on the Ella platform offer a fast, sensitive, and reproducible method for reliable AAV titer measurement with a broader dynamic range than ELISAs.
Protein Expression Potency Assays
With potency being one of the most important CQAs for gene therapy analysis, it is crucial to have a robust assay that provides highly reproducible results.
Traditional methods, such as ELISAs, Western blots and flow cytometry, have limitations, including limited quantitation, large sample volume requirements, and low throughput.
By switching to an automated platform, you can get fast and reproducible protein quantitation that’s simple to transfer to other groups and sites.


Recommended for AAV Potency Analysis: Simple Western™
Simple Western pairs the flexibility and specificity of Western blots with the analytical-grade quantitation of ELISA, making it the ideal system for measuring transgene expression in complex sample types.
AAV Empty/Full Ratio Analysis
The presence of empty or partially filled AAV particles can compromise the purity and safety of the final product in gene therapy.
Traditional methods, such as analytical ultracentrifugation assays (AUC) and ELISAs, are often unreliable, insensitive, and not easily scalable for manufacturing needs.
By transitioning to an automated solution, you can get consistent and sensitive quantification of empty/full capsid ratios, with scalability for adapting to AAV manufacturing workflows.
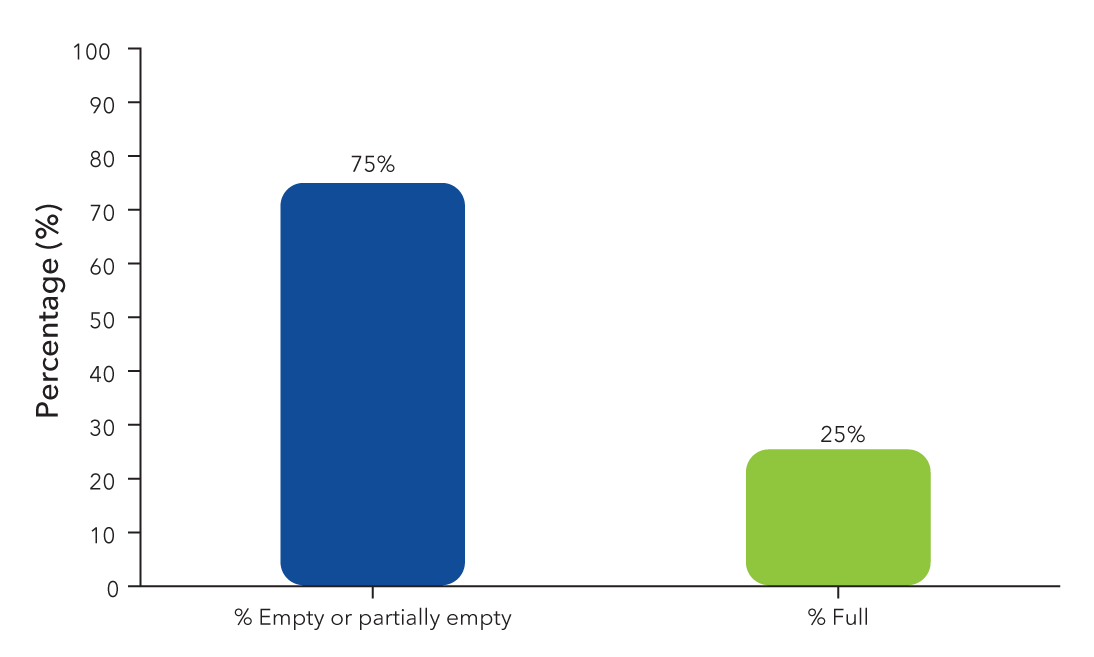
Recommended for AAV Empty/Full Ratio Analysis
Upstream Analysis – Simple Western
Upstream Analysis – Simple Western
For analysis of unpurified samples, Simple Western enables automated AAV empty/full ratio measurements in <6 hours.
Downstream Analysis - Maurice
Downstream Analysis - Maurice
Save time, labor, and sample compared to AUC and get critical capsid content information with ciEF and CE-SDS using Maurice.
AAV Identity Confirmation & Capsid Protein Ratio Analysis
Validating AAV identity is critical for selecting the right vector for targeted gene delivery, as AAV serotypes exhibit different tropisms and immunogenic profiles, as well as individuals having pre-existing immunity to specific serotypes.
As you scale up your workflows, the low-throughput and lengthy processes of SDS-PAGE, Western blotting, and mass spectrometry may cause bottlenecks.
By moving to an automated method, you not only expedite your process, but also receive high resolution and reproducible data using smaller sample volumes.
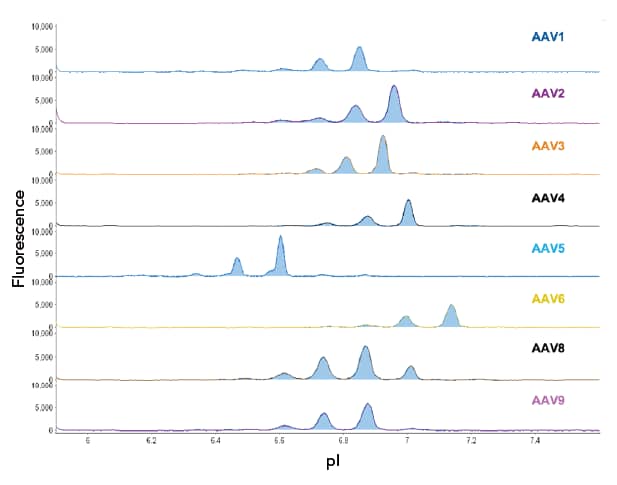
Recommended for AAV Identity and Capsid Protein Ratio Analysis
Crude Sample Analysis - Simple Western
Crude Sample Analysis - Simple Western
For measuring complex samples, such as cell and tissue lysates, Simple Western provides reproducible AAV quantitation with 3 µL of sample required per well.
Pure Sample Analysis - Maurice
Pure Sample Analysis - Maurice
For >70% pure samples, Maurice offers direct, highly sensitive measurements of capsid proteins with method development in a day.
AAV Purity Assays
Detecting and quantifying residual proteins, such as host cell proteins (HCPs) from HEK293 cells or endonucleases from AAV vector preparation, is crucial for ensuring the purity of AAV products.
High sensitivity platforms are necessary to identify low levels of impurities that can impact product efficacy, alter quality, and potentially trigger immune reactions in gene therapy applications.
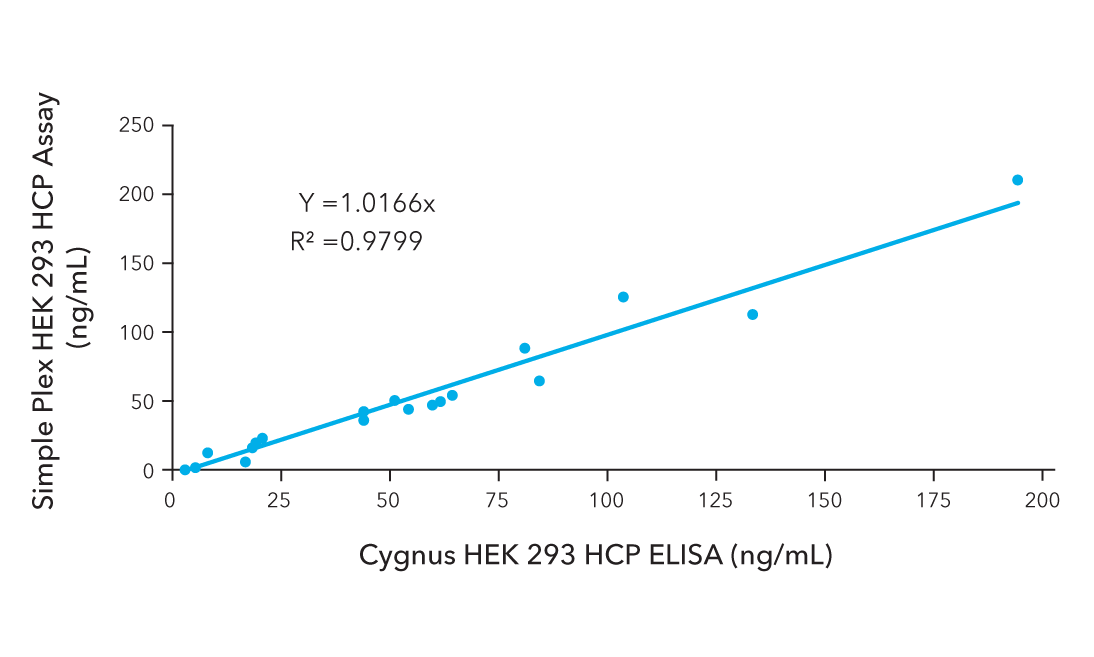

Recommended for AAV Purity Analysis: Simple Plex
Quantify residual endonucleases and HEK 293 HCP impurities in less than 90 minutes with Simple Plex assays. These automated assays ensure precise protein detectability at each stage of the purification process.
AAV Stability Assays
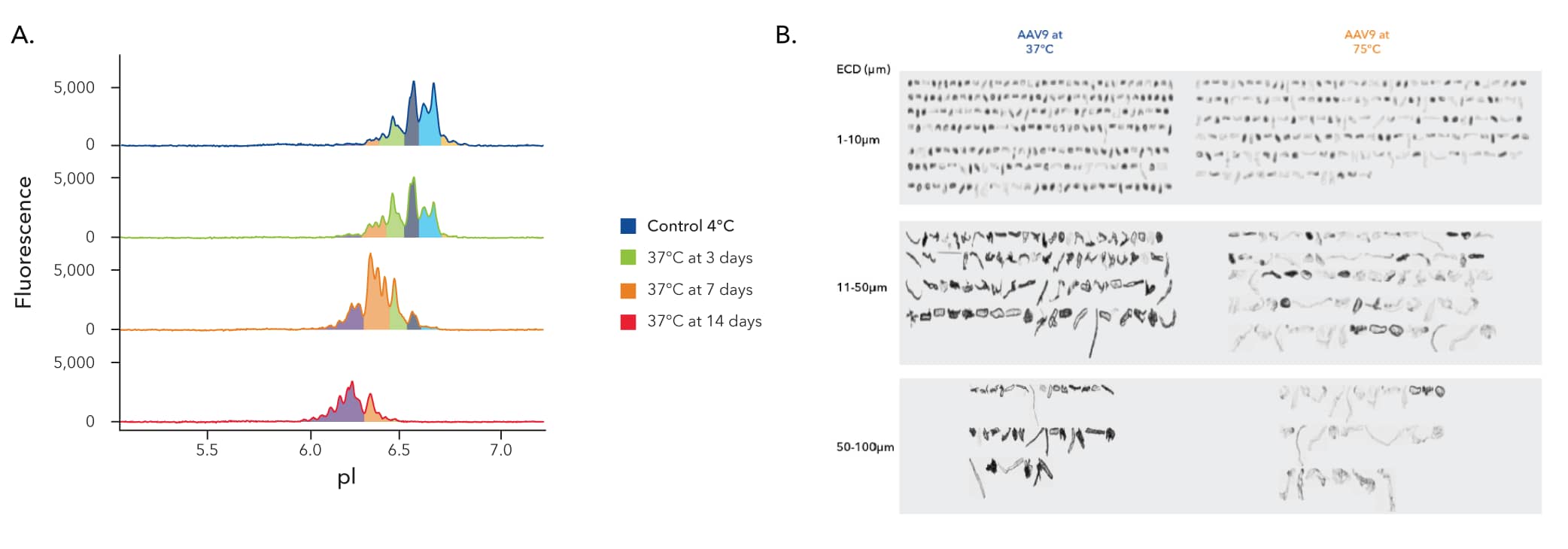
Stability assessment plays a pivotal role in ensuring AAV vector particles maintain their structural and functional integrity over time, minimizing impacts on potency, safety, and efficacy.
Typical methods of AAV stability analysis, such as dynamic light scattering (DLS) and size exclusion chromatography (SEC) are limited by resolution and offer varying degrees of quantification.
By moving to a direct detection method, you can get higher levels of sensitivity in less time, providing robust stability data to simplify regulatory approvals.
Recommended for AAV Stability Analysis
AAV Product Stability: Maurice
AAV Product Stability: Maurice
Get high resolution AAV vector stability data with CVs <5% without dependence on particle size, using icIEF with Maurice.
AAV Aggregation Quantification: MFI™
AAV Aggregation Quantification: MFI™
Identity AAV aggregates and impurities in your samples with higher sensitivity than light obscuration methods using Micro-Flow Imaging™ (MFI)
AAV Vector Biodistribution Analysis
Regulatory agencies strongly recommend biodistribution studies to characterize engineered therapy products.
The RNAscope™ in situ hybridization (ISH) technology is an ideal solution for detecting AAV vector DNA and therapeutic transgene mRNA expression with morphological context, addressing critical questions on tissue biodistribution, cellular tropism, and vector performance in vivo. Easily evaluate dosing, safety and efficacy, and functional markers using RNAscope ISH.

- Characterize tissue biodistribution, cellular tropism, and transduction efficiency of your vector at single-cell resolution in conjunction with cell-type specific markers.
- Measure abundance of AAV+ cells in target tissues and track vector persistence over time.
- Quantify RNA expression of any vector cargo including sequence or codon-optimized human transgenes, CRISPR/Cas9, guide RNAs, or other regulatory non-coding RNAs.
- Demonstrate therapeutic efficacy within vector transduced cells and tissues.
- Easily Scale from small animal models to non-human primates with the capability to easily distinguish human transgenes from homologous host transcripts.
Overview of Multi-Attribute AAV Characterization Platforms
Our hands-free analytical instruments provide multi-faceted CQA analysis to streamline your AAV manufacturing workflows. With automation and scalability, AAV characterization methods can seamlessly transfer across labs and project phases, from discovery to manufacturing. Each of these instruments operates with 21CFR Part 11-compliant software, getting you through regulatory scrutiny and providing consistent results from start to finish.
Platform Specifications |
Simple Western |
Simple Plex |
Maurice |
Main Sample Type |
Virus, cell, and tissue lysates |
Virus lysates |
Virus preparation (>70% purity) |
Assay Type |
CE-SDS + immunoassay |
Sandwich ELISA |
CE-SDS or icIEF |
Detection Modes |
Chemiluminescence, Fluorescence, Total protein stain |
Fluorescence |
Absorbance & native fluorescence |
Sample Volume |
3 µL |
25 µL |
50 µL |
Assay Development |
Single antibody – open platform |
None – fully validated assay kits |
No antibody – open platform |
Quantitative |
Yes |
Yes |
Yes |
Size separation |
Yes |
No |
Yes |
Dynamic Range |
3 to 4 logs |
3 to 5 logs |
3 logs |
Detection Range (GC or VP/mL) |
109 - 1012 |
106 - 1010 |
1011 - 1014 |
Runtime |
3 hours, 5 hours with RePlex™ |
Less than 90 mins |
5 – 35 mins with CE-SDS, 10 – 15 mins with icIEF |
Speak with a Specialist
We are here to help you find the best solution for your AAV characterization needs. Fill out the form and a specialist will be in touch to discuss your requirements.



