Faster instruments are one way to increase efficiency in the lab, but another way is to make them work for you in as many ways as possible. While the Maurice™ system is most powerful for AAV characterization through its charge heterogeneity (icIEF) application, it also enables size separation and analysis through capillary electrophoresis-sodium dodecyl sulfate (CE-SDS). Both applications offer extensive insights into AAV capsid structure and viral stability.
With icIEF, icIEF fractionation, and CE-SDS Maurice platforms can be used to analyze multiple Critical Quality Attributes (CQAs) of AAVs. In their work on developing platform methods for AAV analysis, He et al. demonstrated the robustness of icIEF for monitoring capsid protein deamidation. The study entailed the analysis of deamidation on AAVs with icIEF, alongside the use of orthogonal methods like RC-HPLC and high-resolution LC-MS. The results were comparable across all three methods, highlighting the suitability of icIEF as a GMP-compliant method for routine testing and quality control.
The key benefits of using icIEF as a routine assay are its ease of use, relative cost efficiency, and speed, providing critical insights through high-quality data. The study further illustrated an inverse correlation between deamidation and relative potency. Because deamidation directly impacts the stability of AAV, the study also deemed icIEF as a quick stability-indicating assay1.In addition, another Maurice user presented his work on AAV analysis at the ASGCT conference and the ProteinSimple User Meeting, RTP in 2024. He highlighted his icIEF workflow for assessing deamidation and further demonstrated a link between increased deamidation and potency loss. In another study, heat-stressed AAV samples analyzed on the Maurice system showed marked differences in their charge profiles as the temperature and number of days increased, indicating changes in stability2.
Both icIEF and CE-SDS analyze a host of other AAV CQAs. Charge profiles, when analyzed with both absorbance and native fluorescence detection modes on the Maurice system, provide details on empty vs. full AAV capsids2. CE-SDS, by analyzing protein size, measures the capsid-protein ratio. It also assesses the purity of AAV samples3. Both icIEF and CE-SDS distinguish between various AAV serotypes, thus providing critical information on AAV identity3,4.
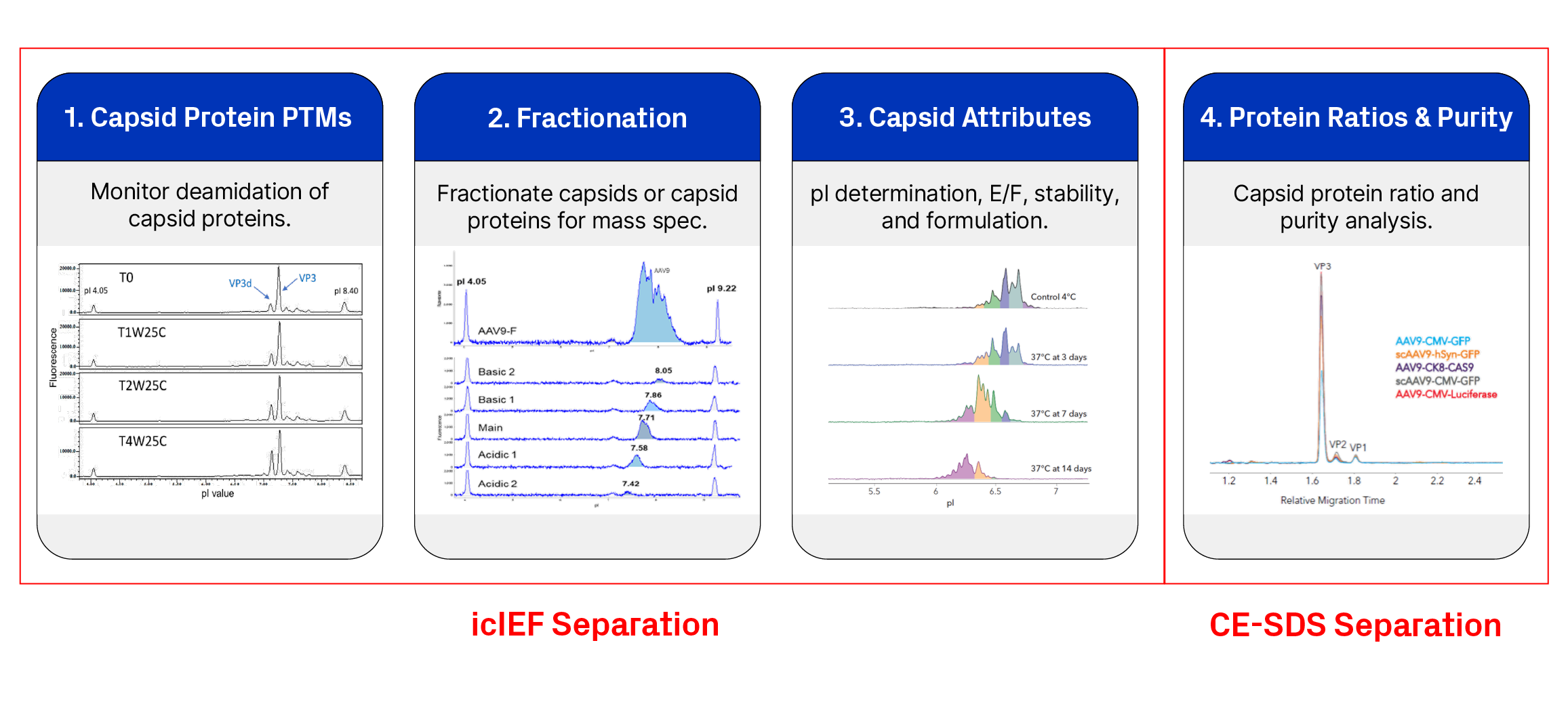
Adding on to existing capabilities, the new MauriceFlex™ system enables icIEF-based fraction collection, thereby allowing the characterization of charge variants with techniques like mass spectrometry. An upcoming publication in the journal of Electrophoresis, titled Enabling icIEF Peak Identification of AAV Capsid Proteins by Fractionation on MauriceFlex™ and Subsequent Analysis by LC-MS, describes a streamlined workflow for the deep characterization of AAV capsids. With the ability to analyze various attributes of AAVs, a single instrument—in this case, Maurice/MauriceFlex —provides immense value in terms of cost, time, and footprint. Learn more about the Maurice platforms.
Request Information: Learn more. Get a quote.
References
- He XZ, Powers TW, Huang S, et al. Development of an icIEF assay for monitoring AAV capsid proteins and application to gene therapy products. Mol Ther Methods Clin Dev. 2023;29:133-144. Published 2023 Mar 10. doi:10.1016/j.omtm.2023.03.002
- Application Note: Assessing Your AAV Product Quality? Get the Confidence You Need With Maurice
- Application Note: Characterize Your Viral Vectors from Discovery to GMP Release with Maurice
- Maurice Spotlight: AAV Analysis Simplified