By Michalina Hanzel, PhD
Astrocytes are a type of macroglia found in the central nervous system that regulate a vast array of essential brain functions, ranging from synaptogenesis, ion homeostasis and maintenance of the blood-brain barrier, to buffering of neurotransmitters and secretion of neuroactive agents. Considering the plethora of roles that astrocytes perform, it’s easy to imagine them as consisting of diverse and distinct subtypes. However, only recently studies have started to characterize the heterogeneity of astrocytes and our understanding of their diversity is still largely unknown, both at the regional (between brain regions) and local (within the same brain region) levels. In a recent review by Khakh & Deneen (2019)1, the authors present a compelling case for protoplasmic astrocyte diversity. They argue that, while a lot of research is still needed, evidence for astrocytic heterogeneity at both the morphological and physiological levels is robust and essential for understanding brain function in health and disease.
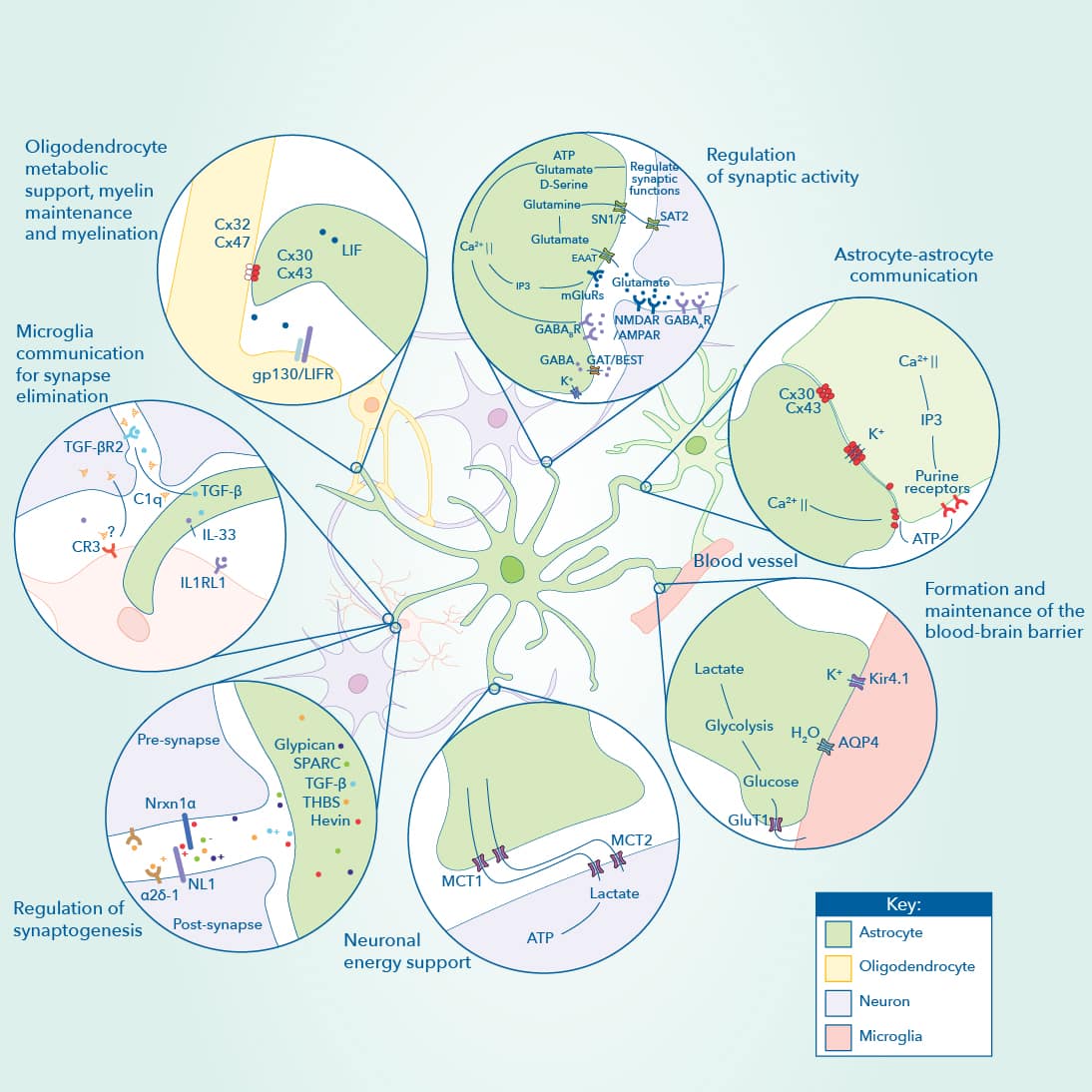
Astrocytes play diverse functions in the central nervous system. Astrocytes form part of tripartite synapses, influencing synaptic activity. Besides their interaction and communication with neurons, astrocytes engage in interactions with each other through gap junctions, modulate the blood-brain barrier, maintain energy homeostasis and also interact with other cell types of the CNS such as oligodendrocytes and microglia. Diagram and legend modified; published in their original form in a collaboration, Astrocyte Development and Function, Pathways and Key Molecular Targets between Benjamin Deneen, PhD., Debosmita Sardar, PhD., and Yi-Ting Cheng, MS., at the Baylor College of Medicine and Novus Biologicals, a Bio-Techne brand.
How do astrocytes differ from each other?
At the level of morphology, researchers who study astrocytes face unique challenges. Neuroscientists typically use morphology, location within the brain, and expression of molecular markers to define specific neuronal populations. However, astrocytes seem more complex than any other brain cell type, and their full morphological complexity has not yet been defined using available methods such as fluorescent and electron microscopy. It is estimated that a single astrocyte, consisting of thousands of tiny processes, can contact up to 100,000 synapses, while simultaneously contacting blood vessels through polarized compartments2. Single astrocytes can thus possess several different end feet structures with very different functions. Currently, however, astrocyte processes are still often viewed as homogenous structures. A more nuanced view is urgently needed to characterize the gross morphology of astrocytes, but also the many compartments within a single cell.
At the physiological level, astrocyte diversity is even less well-characterized. Astrocytes, in contrast to neurons, are electrically silent, and coupled to dozens of other astrocytes, making electrophysiology an impractical tool for their study. However, astrocytes do communicate using changes in Ca2+ ion concentrations or calcium signaling and there are numerous functional roles for these signals that are still being investigated. Recent invention of genetically encoded calcium indicators allows us to directly observe a previously unappreciated richness in astrocyte calcium signaling. Those signals are diverse in their properties, can occur in separate cellular compartments at a time, have different underlying mechanisms and don’t always depend on neuronal activity. Future studies of calcium signaling properties will help reveal astrocyte diversity at a functional level and clarify how different calcium concentration changes mediate their many functions.
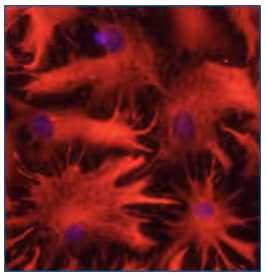
Rat astrocytes in culture were immunostained with 10 µg/mL Sheep Anti-Human GFAP Antigen Affinity-purified Polyclonal Antibody (AF2594) and detected with NorthernLights™ 557-conjugated Anti-Sheep IgG Secondary Antibody (red; NL010). Nuclei were counterstained with DAPI (blue).
Future considerations for astrocyte diversity studies
Classifying astrocyte diversity is extremely important to understand how this cell type shapes and contributes to brain function in health and disease. Multiple, parallel approaches should be used when classifying astrocyte diversity: patch clamping, imaging and pharmacology, morphology, RNA-seq, proteomics, expression validation and bioinformatics. Additional metrics will also be required in future, more nuanced experiments. Importantly, results from studies on astrocytes should only be accepted if the brain region studied is clearly defined in the methods to avoid confounding findings. We now understand that astrocytes are not a homogenous cell type, but clearly defining their diversity will be a big challenge for neuroscientists in the coming years.

Michalina Hanzel, PhD
Postdoctoral Associate at The Rockefeller University
Dr. Hanzel is currently studying synaptic function in the cerebellum to understand neurodevelopmental disorders and has a background in developmental neurobiology, molecular and cell biology.
-
Khakh, B. S., & Deneen, B. (2019) The Emerging Nature of Astrocyte Diversity Annual Review of Neuroscience
-
Chai, H. et al. (2017) Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence Neuron