By Victoria Osinski, PhD
What’s “Natural” About Natural Killer (NK) Cells?
For immunologists, the term cytotoxicity often conjures up images of an army of antigen specific CD8+ T cells deploying to find and kill their target cells. However, natural killer (NK) cells are a distinct subset of cytotoxic immune lymphoid cells with both innate and adaptive immune functions. Innate functions are first-line, non-specific responses to pathogens or damage, while adaptive immunity describes the specific, antibody and cell-mediated responses. Lacking highly-specific antigen recognition receptors, these cells, as the name implies, can also induce antibody-independent killing of target cells and secrete cytokines and other factors that modify the responses of other immune cells.1-3
NK cells express several receptors that bind to MHC class I molecules on surrounding cells, and downstream signaling can either activate or inhibit NK activity depending on signal integration. One class of these MHC class I-binding receptors are known as natural-killer group 2 (NKG2).2-4 NKG2C, -E, -F, and -H are activating receptors which recruit adaptor protein DAP-12, containing the immunoreceptor tyrosine-based activation motif (ITAM). NKG2D, which is only distantly related to the other NKG2 proteins is also activating. NKG2A and -B are inhibitory receptors which contain immunoreceptor tyrosine-based activation motifs (ITIMs).5 Another class of MHC class I-binding receptors are the killer cell immunoglobulin-like receptors (KIRs), which can activate or inhibit NK cell activity, though initially thought to only inhibit it.6
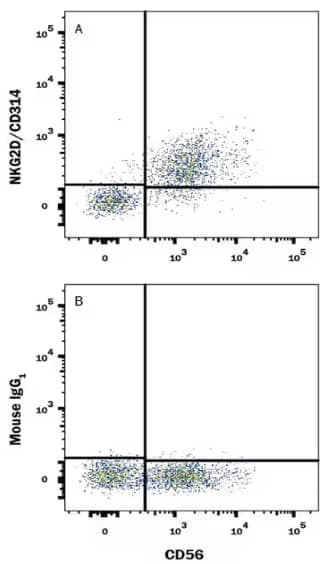
Flow Cytometry analysis of human peripheral blood mononuclear cells stained with Mouse Anti-NCAM‑1/CD56 APC‑conjugated Monoclonal Antibody (Catalog # FAB2408A) and either Mouse Anti-NKG2D/CD314 Monoclonal Antibody (Catalog # MAB139) or Mouse IgG1 Isotype Control (Catalog # MAB002) followed by PE-conjugated anti-Mouse IgG Secondary Antibody (Catalog # F0102B).
Targeting NK Cells For Cancer Treatment
NK cells, and ex vivo modification of NK cells, are a promising set of new tools for cancer immunotherapies. In general, NK cells are considered an optimal cell type for immunotherapy because they can recognize multiple cancer cell types, have a limited lifespan so overactivation is much less likely, and they have limited ability to induce graft-versus-host disease.7,8 Multiple different chimeric antigen receptor (CAR)-NK cell therapies are being developed including anti-CD19 CAR-NK cells to treat acute lymphoblastic leukemia (ALL) and others designed to target either lineage markers, including CD7 and CD33, or tumor-associated antigens, such as MUC1 and HER2.7
As summarized in a review by Frazao et al, the NKG2D-NKG2D-ligand signaling axis is of particular interest in NK cell immunotherapy development. Due to intracellular stress, tumor cells can upregulate NKG2D ligands (NKG2D-L) such as MICA, MICB, and ULBPs, which bind to NKG2D on NK cells and induce cytotoxic responses.7 Interestingly, histone deacetylase (HDAC) inhibitors are emerging as a new anti-cancer drug option to be used in combination with other therapies.9 These drugs can stimulate expression of NKGD-L’s on tumor cells, enhancing their susceptibility to NK cell killing.7 Transducing CAR-NK cells with NKG2D for optimizing solid tumor treatment is currently under investigation to enhance potency of CAR NK cell therapy. However, the great extent to which these proteins contain polymorphisms and allelic variation may present some obstacles.7 Additionally, other therapies such as those inhibiting oncogene-driving mutations can reduce tumor cell proliferation and down-regulate NKG2D-L7, so it is important to characterize the cancer and consider therapy combinations prior to starting treatment.
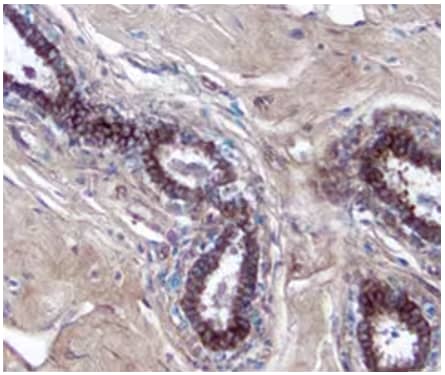
Human breast cancer tissue showing detection of MICA by probing with Goat Anti-Human MICA Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1300), followed by staining with a Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown) and counterstaining with hematoxylin (blue).
Additionally, tumors have been shown to upregulate HLA-G, which binds to the inhibitory receptor KIR2DL4.10 Unlike other HLA class I molecules, HLA-G is not expressed at high levels throughout the body; the exception being in the placenta as a way to promote immune-tolerance during fetal development. Thus, blocking the KIR2DL4-HLA-G signaling access is a promising option for new cancer treatments.10 In some circumstances, HLA-G is not upregulated until advanced stages of tumor growth when effector cell exhaustion is more prevalent, limiting therapeutic efficacy. Nonetheless, HLA-G exhibits lower polymorphism frequencies and its isoforms are characterized. Coupled with detailed tumor diagnostics, KIR2DL4-HLA-G blocking strategies may still serve as excellent therapy options for certain cancers.
Overall, NK cells, with their diverse set of tumor-detecting receptors, shorter life span, and cytotoxic capacity, serve as a promising cell type to use and target with immunotherapies for the treatment of cancer. New approaches to enhance activating- or block inhibitory signals in these cells could revolutionize immunotherapies for both solid tumor and blood cancers.
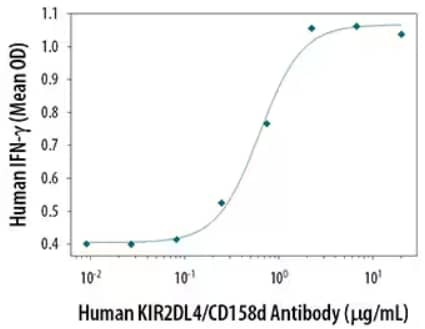
Mouse Anti-Human KIR2DL4/CD158d Monoclonal Antibody (Catalog #MAB2238) stimulates IFN-gamma secretion in NK-92 natural killer lymphoma cell line. Results measured by Quantikine Human IFN‑ gamma ELISA Kit (Catalog # DIF50).

Victoria Osinski, PhD
University of Minnesota Medical School
-
Vivier, E. et al. (2008) Functions of natural killer cells. Nature immunology 9:503-510.
-
Paul, S. et al. (2017) The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Frontiers in immunology 8:1124.
-
Shimasaki, N. et al. (2020) NK cells for cancer immunotherapy Nature reviews. Drug discovery 19:200-218.
-
Long, E. O. et al. (2013) Controlling natural killer cell responses: integration of signals for activation and inhibition. Annual review of immunology 31:227-258.
-
Brostjan, C. et al. (2000) The NKG2 natural killer cell receptor family: comparative analysis of promoter sequences Genes and immunity 1:504-508.
-
Pende, D. et al. (2019) Killer Ig-Like Receptors (KIRs): Their Role in NK Cell Modulation and Developments Leading to Their Clinical Exploitation. Frontiers in immunology 10:1179.
-
Frazao, A. et al. (2019) NKG2D/NKG2-Ligand Pathway Offers New Opportunities in Cancer Treatment Frontiers in immunology 10:661.
-
Xie, G. et al. (2019) CAR-NK cells: A promising cellular immunotherapy for cancer EBioMedicine 59:102975.
-
Eckschlager, T. et al. (2017) Histone Deacetylase Inhibitors as Anticancer Drugs International journal of molecular sciences 18:1414.
-
Loustau, M. et al. (2020) HLA-G Neo-Expression on Tumors Frontiers in immunology 11:1685.