By Christina Towers, PhD.
MAPK Signaling and Disease
Mitogen-activated protein kinases (MAPKs) are serine-threonine kinase signaling molecules that function in a variety of cellular processes including cell differentiation, proliferation, survival, death, and transformation. In mammals, there are 3 subfamilies of MAPKs including ERK, p38, and c-Jun each containing multiple isoforms. The MAPK signaling cascade is mediated by an upstream MAP3K which phosphorylates and activates a MAP2K which in turn activates a MAPK leading to rapid subsequent phosphorylation and activation of critical downstream targets. MAPK signaling has been implicated in a variety of neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, and Amyotrophic lateral sclerosis (ALS) and is thought to play a pathological role in part due to its ability to promote inflammation1.
Connecting Autophagy and Inflammation
It has become widely accepted that inflammation plays a significant role in many neurodegenerative diseases and that both the adaptive and innate immune systems are activated at different stages of disease progression2. The recycling process known as autophagy is another conserved cellular pathway that plays an integral role in the brain and has been implicated in the pathologies of Alzheimer's, Parkinson's and ALS3. Consequently, autophagy and inflammation are intertwined. Autophagy often plays an anti-inflammatory role and regulates innate immunity and inflammasome activity. Inflammatory signals can also activate autophagy. However, it remains unclear how inflammation can evade the negative regulation of autophagy and under what conditions this might occur. Recently, He et. al. identified p38 MAPK as the de-repressive link between inflammation and autophagy4.
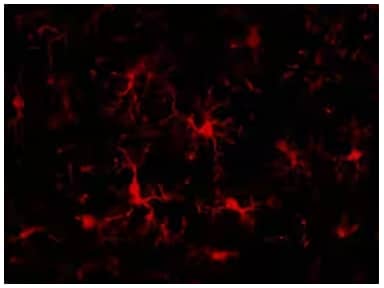
AIF-1/Iba1 protein is a marker of activated microglia. Immunocytochemistry: AIF-1/Iba1 Antibody [NB100-1028] - Analysis of Iba1 in rat brain and spinal cord.
Critical Role of p38 MAPK in Autophagy Inhibition
In a recently published article in the Journal of Cell Biology, the authors show that stimulation of an immune response using Lipopolysaccharides (LPS) represses autophagy specifically in microglial cells but not in other cell types of the brain. The LPS-mediated decrease in autophagy could be reversed with MAPK inhibitors that specifically inhibit p38 phosphorylation linking p38 to this signaling network. The authors go on to provide in depth mechanistic insight showing that p38 MAPK interacts directly with the autophagy promoting kinase ULK1. This interaction leads to ULK1 phosphorylation and inhibition of its kinase activity as well as reduced interaction with its pro-autophagic partner ATG13 in a dose dependent manner. This mechanism is relevant for microglial activation, and they show that pharmacological inhibition of ULK1 or the downstream autophagy protein (ATG5) can activate microglial cells to a similar level as LPS on its own. Moreover, MAPK inhibition blocks LPS mediated microglial activation in both cultured primary cells and in mouse brains.
Significance
These findings directly link MAPK signaling to the intertwined network of autophagy and inflammation and provide new insight into the pathologically relevant inflammatory response that can promote devastating neurodegenerative diseases.

Christina Towers, PhD
University of Colorado (AMC)
Dr. Towers studies the roles of autophagy, apoptosis and cell death in cancer.
-
Kim, E. K. & Choi, E. J. (2010) Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 1802:396-405.
-
Chitnis, T. & Weiner, H. L. (2017) CNS inflammation and neurodegeneration. J Clin Invest 127:3577-3587.
-
Towers, C. G. & Thorburn, A. (2016) Therapeutic Targeting of Autophagy. EBioMedicine
-
He, Y. et al. (2018) p38 MAPK inhibits autophagy and promotes microglial inflammatory responses by phosphorylating ULK1. J Cell Biol