By Jamshed Arslan, Pharm D, PhD
What do nuts, dairy and red meat have in common? In addition to the fact that they are all edible, one of the answers is L-arginine. This amino acid improves the response of T cells against tumor cells, which typically have low concentrations of L-arginine. Increasing L-arginine intra-tumorally, in turn, increases the effect of immunotherapy drugs called immune checkpoint inhibitors (ICIs). To understand ICIs, consider two examples of checkpoint proteins, programmed death-ligand 1 (PD-L1) and Programmed death-1 (PD-1), which are present on tumors and T cells, respectively. Binding of PD-L1 with the receptor PD-1 inhibits T cells from killing cancer cells. Similar to antibodies targeted against PD-L1 or PD-1, ICIs block this binding, allowing T cells to attack the malignant cells. Until recently, there was no means available to continuously enhance L-arginine levels inside tumors. This dilemma was solved when Swiss and American research teams, headed by Dr. Geiger, Dr. Sallusto, and Dr. Lora, engineered a probiotic strain, Escherichia coli Nissle 1917 (ECN), in such a way that the synthetic bacteria could colonize cancers and convert their ammonia waste into L-arginine. Increasing intra-tumoral L-arginine via this synthetic biology technique enhanced T cell infiltration and consequently, synergized the antitumor effect of anti-PD-L1.
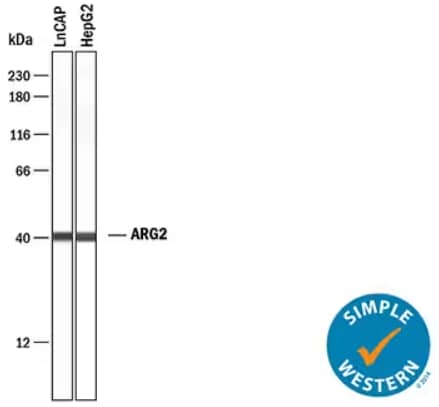
Simple Western lane view analysis depicting lysates from LNCaP human prostate cancer cell line and HepG2 human hepatocellular carcinoma cell line probed with Rabbit Anti-Human Arginase 2/ARG2 Monoclonal Antibody (Catalog #MAB10603). ARG2 is the enzyme responsible for conversion of L-arginine to L-ornithine and urea. A specific band for ARG2 was detected in both sample lysates at approximately 40 kDa (as indicated). The Simple Western experiment was conducted under reducing conditions on a 12-230 kDa separation system.
A Hands-on Approach: Synthesizing L-Arginine-Producing Bacteria
To generate high intra-tumoral L-arginine, the researchers designed tumor-infiltrating ECN bacteria whose L-arginine production was uninhibited by augmented L-arginine levels. To do so, they knocked out the arginine repressor gene (ArgR) and integrated a mutated form of N-acetylglutamate synthase (ArgA) gene into the ECN. In vitro and mass spectrometry-based proteome analyses confirmed an enhanced intra-tumoral production of L-arginine by the engineered L-Arg bacteria. Subsequently, flow cytometry results revealed an increased number of tumor-infiltrating CD4+ and CD8+ T cells.
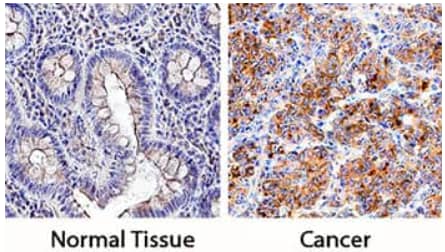
Immersion fixed paraffin-embedded sections of normal human colon (left) and human colon cancer tissue (right) showing PD-L1 expression. PD-L1 was detected in tissue using Goat Anti-Human PD-L1/B7-H1 Polyclonal Antibody (Catalog # AF156) followed by staining with the Anti-Goat HRP-DAB IHC Cell & Tissue Staining Kit (brown) and counterstaining with hematoxylin (blue). Specific PD-L1/B7-H1 staining was localized to cell membranes and cytoplasm.
Synergism of Intra-Tumoral L-Arginine Abundance with Anti-PD-L1 Therapy
After demonstrating a workable bacterial model for intra-tumoral L-arginine production, the team explored the additive effects of L-arginine with PD-L1 blockade. To investigate the combinatorial effects, L-Arg bacteria were intra-tumorally injected with or without PD-L1 antibodies into mice bearing subcutaneous MC38 tumors. The combined therapy completely eradicated the tumors in 74% of such mice. Repeating the same experiment in mice with organized lymphoid structures but lacking all T cells failed to reduce the cancer growth. These results indicated that the L-Arg and anti-PD-L1 synergism in dependent upon T cells. Further experimentation revealed that since L-arginine promotes a memory phenotype in T cells, when mice that had their tumors receded were then challenged with subcutaneous MC38 tumor injection, no malignant growth occurred. This long-term anti-tumor memory was shown to be specific to MC38 tumors as challenge with another cancer cell line could not protect the mice from malignant growth.
Since intra-tumoral L-Arg injection is limited in its applications, the team administered L-Arg intravenously with anti-PD-L1 to MC38-bearing mice to target non-accessible tumors. This intervention reduced the growth of relatively large tumors and 40% of the mice showed a complete rejection of cancer. These results were reproducible with other cell lines as well. In sum, combined therapy with L-Arg and PD-L1 antibodies is a feasible approach to tackle multiple cancers.
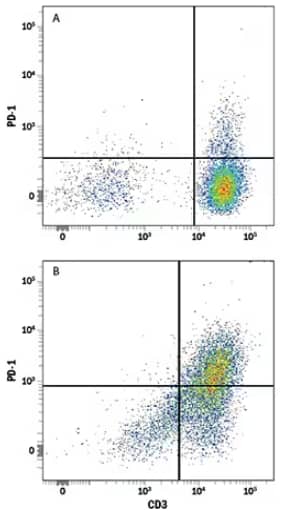
Flow Cytometry analysis showing human peripheral blood mononuclear cells (PBMCs) either (A) untreated or (B) treated with Phytohemagglutinin (PHA), which stimulates T cell activation, and stained using Mouse Anti-Human PD‑1 Monoclonal Antibody (Catalog # MAB10861) followed by Allophycocyanin (APC)-conjugated Goat Anti-Mouse IgG Secondary Antibody (Catalog # F0101B) and Phycoerythrin (PE)‑conjugated Mouse Anti- Human CD3 epsilon Monoclonal Antibody (UCHT1) (Catalog # FAB100P). Quadrants were set based on staining with Mouse IgG Isotype Control Antibody (Catalog # MAB002).
Way Forward
The fueling of immune cells by synthetic bacteria-induced recycling of tumor waste is a novel approach to boost immunotherapy. Based on the current study future directions include determining the mechanism of action of L-arginine, establishing types of tumors responsive to L-Arg, and expanding upon the safety and efficacy of intravenous L-Arg. Overall, this seminal study and its results highlight the need for clinical evaluation of such synthetic biotic medicines as immunotherapy in various types of cancers.

Jamshed Arslan, Pharm D, PhD
Dr Arslan is an Assistant Professor at Salim Habib University (formerly, Barrett Hodgson University), Pakistan. His interest lies in neuropharmacology and preparing future pharmacists.
-
Canale, F.P. et al. (2021) Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature 7882:662. PMID: 34616044.