Analytical Validation of a PCR/CE Assay that Phases SNPs with CAG-Expanded Alleles for Selecting Huntington Disease Patients for Allele-selective Treatments
by Ashima Sharma, Walairat Laosinchai-Wolf, Erika Mitchen, Deepa Eveleigh, Kortney Wilkinson-Busha. Asuragen, a Bio-Techne brand; Wave Life Sciences USA, Inc.
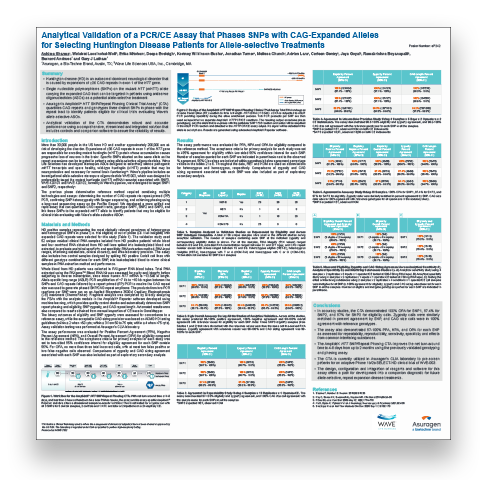
Scientific Meeting PostersHuntington's Disease (HD) is an autosomal dominant neurological disorder that is caused by expansions of >36 CAG repeats in exon 1 of the HTT gene.
- Single nucleotide polymorphisms (SNPs) on the mutant HTT (mHTT) allele carrying the expanded CAG tract can be targeted in patients using antisense oligonucleotides (ASOs) as a potential allele-selective treatment.
- Asuragen’s AmplideX® HTT SNP/Repeat Phasing Clinical Trial Assay* (CTA) quantifies CAG repeats and genotypes three distinct SNPs in phase with the repeat tract to identify patients eligible for clinical trials evaluating Wave’s allele-selective ASOs.
- Analytical validation of the CTA demonstrates robust and accurate performance using a comprehensive, streamlined and integrated solution that includes controls and companion software to assure the reliability of results.