Analysis of Rituximab by Maurice
Introduction
Rituximab is a biological drug used to treat certain cancers and autoimmune diseases. It is one of the earliest antibody-based therapies available for cancer treatment (approved in 1997), and is considered an essential medicine by the World Health Organization1.
Maurice icIEF Method
Carboxypeptidase B (CpB) treatment: Rituximab was diluted to 1.0 mg/mL in water prior to CpB digestion. CpB (1 mg/mL stock solution) was added at a ratio of 1:100 (CpB to sample) and incubated at 37 °C for 20 minutes and then placed on ice. CpB was obtained from Sigma-Aldrich (PN C9584).
Sample preparation: Rituximab was diluted to 0.2 mg/mL in the ampholyte solution.
Ampholyte solution: Pharmalytes 8–10.5 (3%) and 5–8 (1%) containing 3.2 M urea, 5 mM IDA and10 mM arginine. pI markers: 5.85 and 10.17.
Running conditions: 1 minute at 1500 V, then 8 minutes at 3000 V.
Imaging: Absorbance and fluorescence.
Recombinant: The rituximab recombinant was acquired from R&D Systems, PN MAB9575.
Results
To compare rituximab to a recombinant and two biosimilars, we used the icIEF platform method described above to monitor charge heterogeneity by absorbance (Figure 1) and by native fluorescence (Figure 2) detection. These analyses revealed that the innovator, recombinant, and biosimilar #1 are similar in charge heterogeneity and purity. Biosimilar #2, however, had a larger peak area percentage in the basic region (Figure 1, 2). To evaluate if this large peak area percentage in the basic region is due to the presence of lysine variants, the antibodies were treated with CpB before analysis on icIEF. Indeed, treatment with CpB had a dramatic effect on the peak area in the basic region of biosimilar #2, resulting in a >17% decrease, while it had very little effect on the basic region of the others (≤2% decrease in peak area). This indicates that biosimlar #2 contains terminal-lysine-containing variants that are not present in the other samples. Furthermore, it is reasonable to speculate that the two basic peaks in the untreated sample of biosimilar #2 are two different terminal-lysine-containing variants because these two peaks disappear following CpB treatment. Because lysine modification can affect antibody function in vivo2, these variants are important to monitor during antibody process development.
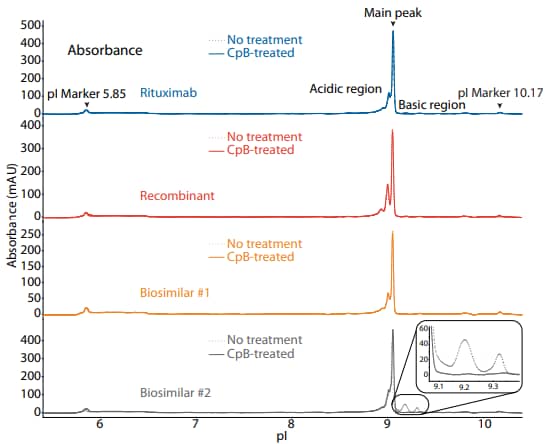
Figure 1. icIEF absorbance (top) and peak area percentages (bottom) of rituximab, a recombinant, and two biosimilars.
| Sample | Acidic Region | Main Peak | Basic Region | Δ Basic Region | |
|---|---|---|---|---|---|
|
Rituximab |
No treatment | 36.1 | 62.7 | 1.2 | N/A |
| CpB-treated | 37.1 | 62.9 | 0.0 | -1.2 | |
|
Recombinant |
No treatment | 44.9 | 53.9 | 1.2 | N/A |
| CpB-treated | 45.2 | 54.1 | 0.7 | -0.5 | |
|
Biosimilar #1 |
No treatment | 37.8 | 61.6 | 0.6 | N/A |
| CpB-treated | 38.4 | 61.6 | 0.0 | -0.6 | |
|
Biosimilar #2 |
No treatment | 27.9 | 54.0 | 18.0 | N/A |
| CpB-treated | 35.1 | 64.7 | 0.2 | -17.8 | |
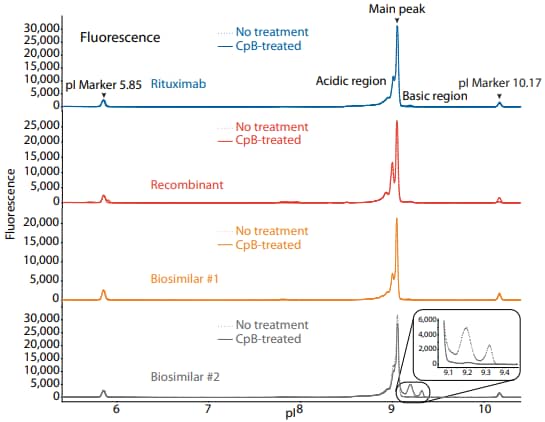
Figure 2. icIEF fluorescence (top) and peak area percentages (bottom) of rituximab, a recombinant, and two biosimilars.
| Sample | Acidic Region | Main Peak | Basic Region | Δ Basic Region | |
|---|---|---|---|---|---|
|
Rituximab |
No treatment | 43.0 | 54.8 | 2.2 | N/A |
| CpB-treated | 44.5 | 55.3 | 0.2 | -2.0 | |
|
Recombinant |
No treatment | 49.1 | 48.7 | 2.2 | N/A |
| CpB-treated | 49.0 | 49.3 | 1.6 | -0.6 | |
|
Biosimilar #1 |
No treatment | 41.7 | 56.7 | 1.6 | N/A |
| CpB-treated | 41.6 | 57.9 | 0.5 | -1.1 | |
|
Biosimilar #2 |
No treatment | 31.0 | 50.4 | 18.6 | N/A |
| CpB-treated | 40.5 | 58.8 | 0.7 | -17.9 | |
Maurice CE-SDS Method
Sample preparation: Rituximab was diluted to 1 mg/mL with 1X Sample Buffer prior to treatment for 10 minutes at 70 °C in the presence of either 11.5 mM IAM (nonreducing) or 650 nM β-ME (reducing).
Running conditions: Samples were injected for 20 seconds at 4600 V, followed by a 25-minute separation (reducing) or a 35-minute separation (non-reducing) at 5750 V.
Results
Rituximab, a recombinant, and two biosimilars were analyzed on CE-SDS platform method under reducing (Figure 3) and non-reducing (Figure 4) conditions, resulting in comparable purity
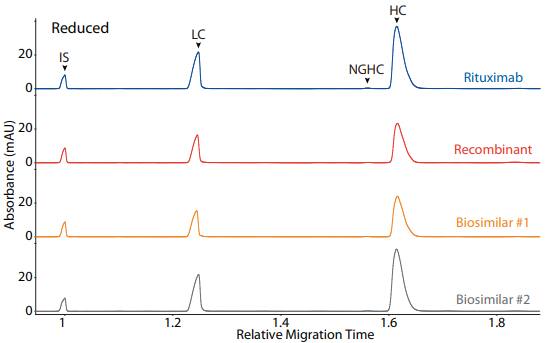
Figure 3. CE-SDS reduced (top) and peak area percentages (bottom) of rituximab, a recombinant, and two biosimilars. (IS) Internal standard. (LC) Light chain. (NGHC) Non-glycosylated heavy chain. (HC) Heavy chain.
| Sample | LC | NGHC | HC | Other |
|---|---|---|---|---|
| Rituximab | 32.6 | 0.4 | 66.7 | 0.3 |
| Recombinant | 34.9 | 0.2 | 63.9 | 0.9 |
| Biosimilar #1 | 32.4 | 0.2 | 67.1 | 0.2 |
| Biosimilar #2 | 32.8 | 0.2 | 66.8 | 0.2 |
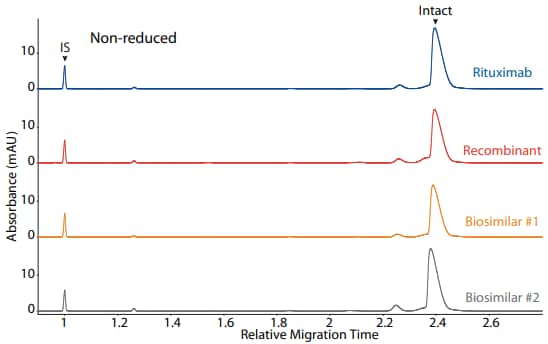
Figure 4. CE-SDS non-reduced (top) and peak area percentages (bottom) of rituximab, a recombinant, and two biosimilars. (IS) Internal standard. (NG) Non-glycosylated.
| Sample | Other | NG | Intact |
|---|---|---|---|
| Rituximab | 6.3 | 0.0 | 93.6 |
| Recombinant | 9.4 | 6.0 | 84.6 |
| Biosimilar #1 | 6.3 | 0.0 | 93.8 |
| Biosimilar #2 | 9.7 | 0.0 | 90.2 |
References
- Essential medicines for cancer: WHO recommendations and national priorities, J Robertson, R Barr, L Shulman, G Forte and N Magrini, Bulletin of the World Health Organization, 2016; 94:735–42.
- Human IgG is produced in a pro-form that requires clipping of C-terminal lysines for maximal complement activation, E van den Bremer, F Beurskens, M Voorhorst, P Engelberts, R de Jong, B van der Boom, E Cook, M Lindorfer, R Taylor, P van Berkel and P Parren, mAbs, 2015; 7:672–80.