Adenosylhomocysteinase/AHCY Products
Human S-Adenosylhomocysteinase (AHCY) is a cytoplasmic tetramer with a tightly bound NAD co-factor for each subunit. It is the only known enzyme to catalyze the breakdown of S-adenosylhomocysteine (AdoHcy) to homocysteine and adenosine. AdoHcy hydrolysis is a reversible reaction with an equilibrium favoring AdoHcy formation, but hydrolysis prevails under physiological conditions due to the rapid removal of adenosine and homocysteine. Thus, AHCY's activity in mammals is directly related to homocysteine level, an independent risk factor for vascular disease. It also functions as a regulator of biological transmethylation by controlling the concentration of AdoHcy, a potent competitive inhibitor of all S-adonosyl-L-methionine methyltransferases. A mutation in the human AHCY results in AHCY deficiency with increase of plasma creatine kinase, methionine, S-adenosylmethionine and AdoHcy, delay of myelination, myopathy and psychomotor retardation.
11 results for "Adenosylhomocysteinase/AHCY" in Products
11 results for "Adenosylhomocysteinase/AHCY" in Products
Adenosylhomocysteinase/AHCY Products
Human S-Adenosylhomocysteinase (AHCY) is a cytoplasmic tetramer with a tightly bound NAD co-factor for each subunit. It is the only known enzyme to catalyze the breakdown of S-adenosylhomocysteine (AdoHcy) to homocysteine and adenosine. AdoHcy hydrolysis is a reversible reaction with an equilibrium favoring AdoHcy formation, but hydrolysis prevails under physiological conditions due to the rapid removal of adenosine and homocysteine. Thus, AHCY's activity in mammals is directly related to homocysteine level, an independent risk factor for vascular disease. It also functions as a regulator of biological transmethylation by controlling the concentration of AdoHcy, a potent competitive inhibitor of all S-adonosyl-L-methionine methyltransferases. A mutation in the human AHCY results in AHCY deficiency with increase of plasma creatine kinase, methionine, S-adenosylmethionine and AdoHcy, delay of myelination, myopathy and psychomotor retardation.
| Reactivity: | Human |
| Details: | Sheep IgG Polyclonal |
| Applications: | WB, Simple Western |
Recombinant Monoclonal Antibody
| Reactivity: | Human, Mouse, Rat |
| Details: | Rabbit IgG Monoclonal Clone #SR1809 |
| Applications: | WB |
Recombinant Monoclonal Antibody
| Reactivity: | Human, Mouse, Rat |
| Details: | Rabbit IgG Monoclonal Clone #4E9H10 |
| Applications: | WB, IP |
| Reactivity: | Human |
| Details: | Mouse IgG1 kappa Monoclonal Clone #M2 |
| Applications: | WB, ELISA |
| Applications: | ELISA |
| Reactivity: | Human, Mouse |
| Details: | Rabbit IgG Polyclonal |
| Applications: | WB |
Recombinant monoclonal antibody expressed in HEK293F cells
| Reactivity: | Human |
| Details: | Rabbit IgG Monoclonal Clone #13H8 |
| Applications: | WB, ELISA |
| Applications: | WB |
EZH2 histone methyltransferase inhibitor
| Alternate Names: | DZNep,NSC 617989 |
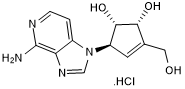
| Chemical Name: | (1S,2R,5R)-5-(4-Amino-1H-imidazo[4,5-c]pyridin-1-yl)-3-(hydroxymethyl)-3-cyclopentene-1,2-diol hydrochloride |
| Purity: | ≥98% (HPLC) |
| Source: | E. coli |
| Accession #: | P23526 |
| Applications: | EnzAct |
3-Deazaneplanocin A hydrochloride synthesized to Ancillary Material Grade
| Chemical Name: | (1S,2R,5R)-5-(4-Amino-1H-imidazo[4,5-c]pyridin-1-yl)-3-(hydroxymethyl)-3-cyclopentene-1,2-diol hydrochloride |
| Purity: | ≥98% |



![Western Blot: Adenosylhomocysteinase/AHCY Antibody (SR1809) [NBP3-22403] -](https://resources.bio-techne.com/images/products/nbp3-22403_rabbit-adenosylhomocysteinase-ahcy-mab-sr1809-5720231595521.jpg)
![Western Blot: Adenosylhomocysteinase/AHCY Antibody (4E9H10) [NBP3-16147] Western Blot: Adenosylhomocysteinase/AHCY Antibody (4E9H10) [NBP3-16147]](https://resources.bio-techne.com/images/products/Adenosylhomocysteinase-AHCY-Antibody-4E9H10-Western-Blot-NBP3-16147-img0002.jpg)
![Western Blot: Adenosylhomocysteinase/AHCY Antibody (M2) [H00000191-M09] Western Blot: Adenosylhomocysteinase/AHCY Antibody (M2) [H00000191-M09]](https://resources.bio-techne.com/images/products/Adenosylhomocysteinase-AHCY-Antibody-M2-Western-Blot-H00000191-M09-img0005.jpg)
![ELISA: Human Adenosylhomocysteinase/AHCY - Ready-To-Use ELISA Kit (Colorimetric) [NBP3-31048] - Human Adenosylhomocysteinase/AHCY - Ready-To-Use ELISA Kit (Colorimetric)](https://resources.bio-techne.com/images/products/nbp3-31048_human-adenosylhomocysteinase-ahcy-ready-to-use-elisa-kit-206202415314825.png)
![Western Blot: Adenosylhomocysteinase/AHCY Antibody [NBP1-55016] Western Blot: Adenosylhomocysteinase/AHCY Antibody [NBP1-55016]](https://resources.bio-techne.com/images/products/Adenosylhomocysteinase-AHCY-Antibody-Western-Blot-NBP1-55016-img0003.jpg)
![Western Blot: Adenosylhomocysteinase/AHCY Antibody (13H8) [NBP3-26516] - Adenosylhomocysteinase/AHCY Antibody (13H8)](https://resources.bio-techne.com/images/products/nbp3-26516_rabbit-adenosylhomocysteinase-ahcy-mab-13h8-282202411363022.jpg)
![Western Blot: Adenosylhomocysteinase/AHCY Overexpression Lysate [NBL1-07402] Western Blot: Adenosylhomocysteinase/AHCY Overexpression Lysate [NBL1-07402]](https://resources.bio-techne.com/images/products/S-Adenosylhomocysteine-Hydrolase-Overexpression-Lysate-Adult-Normal-Western-Blot-NBL1-07402-img0002.jpg)