Glucocorticoid Receptor Agonists Products
Glucocorticoid Receptor (GR), also known as NR3C1, is a member of the NR3 family of nuclear receptors. It mediates the physiological and pharmacological actions of glucocorticoids. GR is a modular protein that is composed of three major domains: a N-terminal transactivation domain (NTD, amino acids [aa] 1-421); a central DNA-binding domain (DBD, aa 422-486); and a C-terminal ligand-binding domain (LBD, aa 528-777). A flexible region, termed hinge region, lies between the DBD and LBD (aa 487-527).
The protein also contains two zinc finger motifs in the DBD (aa 421-441 and 457-476), which recognize and bind target DNA sequences called glucocorticoid-responsive elements (GREs), and two nuclear localization sequences (NLS) found at the junction of the DBD and hinge region, and within the LBD. Two receptor isoforms, termed GR alpha and GR beta, exist due to alternative splicing. GR alpha is the classic GR protein that mediates glucocorticoid actions. Eight different GR alpha subtypes exist due to alternative translation initiation. GR beta, which resides constitutively in the nucleus, does not bind to glucocorticoids, but instead, appears to function as a dominant negative inhibitor to GR alpha. Human GR alpha is 777 aa in length with a predicted molecular length of approximately 85.7 kDa. It shares 89% and 88% aa sequence identity with the mouse and rat orthologs, respectively.
GR is expressed in nearly every cell of the body, and thus, facilitates a vast array of physiological functions including regulation of metabolism, modulation of immune responses, and mediation of short- and long-term adaptations following stress exposure. In the absence of its ligand, GR alpha resides in cytoplasm as part of a large multi-protein complex that includes HSP90, HSP70/HSPA1A, p23, FKBP51, and FKBP52. Upon ligand binding, GR alpha undergoes a conformational change that results in the dissociation of the associated proteins and exposure of the 2 NLSs. GR alpha subsequently translocates into the nucleus where it binds to GREs in target genes and regulates their expression. GR alpha can also elicit rapid cellular responses via activating a number of signaling proteins including AKT, PI 3-Kinase, and MAPKs.
4 results for "Glucocorticoid Receptor Agonists" in Products
4 results for "Glucocorticoid Receptor Agonists" in Products
Glucocorticoid Receptor Agonists Products
Glucocorticoid Receptor (GR), also known as NR3C1, is a member of the NR3 family of nuclear receptors. It mediates the physiological and pharmacological actions of glucocorticoids. GR is a modular protein that is composed of three major domains: a N-terminal transactivation domain (NTD, amino acids [aa] 1-421); a central DNA-binding domain (DBD, aa 422-486); and a C-terminal ligand-binding domain (LBD, aa 528-777). A flexible region, termed hinge region, lies between the DBD and LBD (aa 487-527).
The protein also contains two zinc finger motifs in the DBD (aa 421-441 and 457-476), which recognize and bind target DNA sequences called glucocorticoid-responsive elements (GREs), and two nuclear localization sequences (NLS) found at the junction of the DBD and hinge region, and within the LBD. Two receptor isoforms, termed GR alpha and GR beta, exist due to alternative splicing. GR alpha is the classic GR protein that mediates glucocorticoid actions. Eight different GR alpha subtypes exist due to alternative translation initiation. GR beta, which resides constitutively in the nucleus, does not bind to glucocorticoids, but instead, appears to function as a dominant negative inhibitor to GR alpha. Human GR alpha is 777 aa in length with a predicted molecular length of approximately 85.7 kDa. It shares 89% and 88% aa sequence identity with the mouse and rat orthologs, respectively.
GR is expressed in nearly every cell of the body, and thus, facilitates a vast array of physiological functions including regulation of metabolism, modulation of immune responses, and mediation of short- and long-term adaptations following stress exposure. In the absence of its ligand, GR alpha resides in cytoplasm as part of a large multi-protein complex that includes HSP90, HSP70/HSPA1A, p23, FKBP51, and FKBP52. Upon ligand binding, GR alpha undergoes a conformational change that results in the dissociation of the associated proteins and exposure of the 2 NLSs. GR alpha subsequently translocates into the nucleus where it binds to GREs in target genes and regulates their expression. GR alpha can also elicit rapid cellular responses via activating a number of signaling proteins including AKT, PI 3-Kinase, and MAPKs.
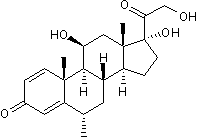
Glucocorticoid receptor agonist
| Chemical Name: | 11β,17β,21-Trihydroxy-6α-methyl-1,4-pregnadiene-3,20-dione |
| Purity: | ≥99% (HPLC) |
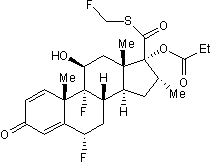
Selective high affinity glucocorticoid receptor agonist
| Chemical Name: | (6α,11β,16α,17α)-6,9-Difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)androsta-1,4-diene-17-carbothioic acid fluoromethyl ester |
| Purity: | ≥98% (HPLC) |
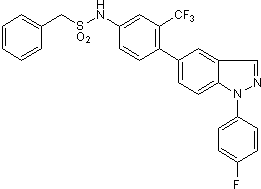
Glucocorticoid receptor agonist
| Chemical Name: | N-[4-[1-(4-Fluorophenyl)-1H-indazol-5-yl-3-(trifluoromethyl)phenyl]benzenesulfonamide |
| Purity: | ≥98% (HPLC) |
Endogenous glucocorticoid
| Chemical Name: | (11β)-11,21-Dihydroxypregn-4-ene-3,20-dione |
| Purity: | ≥99% (HPLC) |