PLC-beta 1 Products
Phosphoinositide-specific Phospholipase C (PLC) is an essential signal transduction molecule that catalyzes the hydrolysis of the cellular membrane phospholipid Phosphatidylinositol 4,5-bisphosphate (PIP2) to generate the second messengers Inositol 1,4,5-trisphosphate (IP3) and Diacylglycerol (DAG). IP3 and DAG then initiate the release of calcium from intracellular stores and the activation of Protein Kinase C, respectively. Currently, thirteen PLC isozymes have been identified and are divided into six PLC subfamilies: PLC-beta, -delta, -epsilon, -eta, -gamma, and –zeta. All PLC isozymes are multi-domain molecules that contain several conserved structural features. These features include the catalytic X and Y domains, EF hand calcium-binding motifs, and an N-terminal pleckstrin homology (PH) and a C-terminal C2 domain, both of which function as membrane-phospholipid binding regions. The PLC-beta subfamily also contains an extra 450 amino acids (aa) in the C-terminus that are thought to be critical for association with G proteins.
PLC-beta 1 is one member of the PLC-beta subfamily. It is 1216 aa in length with a predicted molecular weight of 138.6 kDa. Human PLC-beta 1 shares 97% aa sequence identity with the mouse and rat orthologs. This protein is widely expressed with the highest expression levels being found in the cerebral cortex and hippocampus. PLC-beta 1 exists as two alternative splice variants, PLC-beta 1a and PLC-beta 1b. Both transcripts contain a putative nuclear localization sequence in their C-terminus; however, PLC-beta 1a also has a C-terminal nuclear export sequence. As a result, PLC-beta 1b is mainly localized to the nucleus while PLC-beta 1a can be found within both the cytoplasm and nucleus. PLC-beta 1 is primarily activated by the alpha subunit of Gq/11 proteins and, to a lesser extent, by G protein beta-gamma subunits. Defects in PLC-beta 1 expression have been associated with early-onset epileptic encephalopathy, bipolar disorder, and in the progression of myelodysplastic syndrome to acute myeloid leukemia.
10 results for "PLC-beta 1" in Products
10 results for "PLC-beta 1" in Products
PLC-beta 1 Products
Phosphoinositide-specific Phospholipase C (PLC) is an essential signal transduction molecule that catalyzes the hydrolysis of the cellular membrane phospholipid Phosphatidylinositol 4,5-bisphosphate (PIP2) to generate the second messengers Inositol 1,4,5-trisphosphate (IP3) and Diacylglycerol (DAG). IP3 and DAG then initiate the release of calcium from intracellular stores and the activation of Protein Kinase C, respectively. Currently, thirteen PLC isozymes have been identified and are divided into six PLC subfamilies: PLC-beta, -delta, -epsilon, -eta, -gamma, and –zeta. All PLC isozymes are multi-domain molecules that contain several conserved structural features. These features include the catalytic X and Y domains, EF hand calcium-binding motifs, and an N-terminal pleckstrin homology (PH) and a C-terminal C2 domain, both of which function as membrane-phospholipid binding regions. The PLC-beta subfamily also contains an extra 450 amino acids (aa) in the C-terminus that are thought to be critical for association with G proteins.
PLC-beta 1 is one member of the PLC-beta subfamily. It is 1216 aa in length with a predicted molecular weight of 138.6 kDa. Human PLC-beta 1 shares 97% aa sequence identity with the mouse and rat orthologs. This protein is widely expressed with the highest expression levels being found in the cerebral cortex and hippocampus. PLC-beta 1 exists as two alternative splice variants, PLC-beta 1a and PLC-beta 1b. Both transcripts contain a putative nuclear localization sequence in their C-terminus; however, PLC-beta 1a also has a C-terminal nuclear export sequence. As a result, PLC-beta 1b is mainly localized to the nucleus while PLC-beta 1a can be found within both the cytoplasm and nucleus. PLC-beta 1 is primarily activated by the alpha subunit of Gq/11 proteins and, to a lesser extent, by G protein beta-gamma subunits. Defects in PLC-beta 1 expression have been associated with early-onset epileptic encephalopathy, bipolar disorder, and in the progression of myelodysplastic syndrome to acute myeloid leukemia.
| Reactivity: | Human, Mouse |
| Details: | Sheep IgG Polyclonal |
| Applications: | WB |
| Reactivity: | Human, Rat |
| Details: | Rabbit IgG Polyclonal |
| Applications: | IHC, WB |
Recombinant Monoclonal Antibody
| Reactivity: | Human, Mouse, Rat |
| Details: | Rabbit IgG Monoclonal Clone #S03-6E6 |
| Applications: | WB |
| Reactivity: | Human, Mouse, Rat |
| Details: | Rabbit IgG Polyclonal |
| Applications: | IHC, WB |
| Applications: | WB, ELISA, MA, AP, PAGE |
| Reactivity: | Human, Mouse |
| Details: | Rabbit IgG Polyclonal |
| Applications: | WB |
| Applications: | WB |
| Applications: | AC |
| Applications: | AC |
Phospholipase C activator
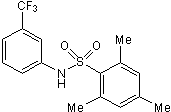
| Chemical Name: | 2,4,6-Trimethyl-N-[3-(trifluoromethyl)phenyl]benzenesulfonamide |
| Purity: | ≥98% (HPLC) |



![Western Blot: PLC-beta 1 Antibody [NBP2-38220] Western Blot: PLC-beta 1 Antibody [NBP2-38220]](https://resources.bio-techne.com/images/products/PLC-beta-1-Antibody-Western-Blot-NBP2-38220-img0002.jpg)
![Western Blot: PLC-beta 1 Antibody (S03-6E6) [NBP3-19739] Western Blot: PLC-beta 1 Antibody (S03-6E6) [NBP3-19739]](https://resources.bio-techne.com/images/products/PLC-beta-1-Antibody-S03-6E6-Western-Blot-NBP3-19739-img0001.jpg)
![Western Blot: PLC-beta 1 AntibodyBSA Free [NBP2-93680] Western Blot: PLC-beta 1 AntibodyBSA Free [NBP2-93680]](https://resources.bio-techne.com/images/products/PLC-beta-1-Antibody-Western-Blot-NBP2-93680-img0005.jpg)
![Western Blot: PLC-beta 1 Antibody [NBP1-58257] Western Blot: PLC-beta 1 Antibody [NBP1-58257]](https://resources.bio-techne.com/images/products/PLC-beta-1-Antibody-Western-Blot-NBP1-58257-img0003.jpg)
![Western Blot: PLC-beta 1 Overexpression Lysate [NBP2-04807] Western Blot: PLC-beta 1 Overexpression Lysate [NBP2-04807]](https://resources.bio-techne.com/images/products/Phospholipase-C-beta-1-Overexpression-Lysate-Adult-Normal-Western-Blot-NBP2-04807-img0001.jpg)