IHC Detection
Chromogenic Detection vs. Fluorescent Detection | Fluorescent Detection | Chromogenic Detection | Signal Amplification Using Chromogenic Detection | Signal Amplification Using Chromogenic Detection
Immunohistochemistry (IHC) Detection
Direct or indirect detection methods can be used to produce a fluorescent or chromogenic signal for protein detection. In direct detection, the primary antibody specific for the target molecule is directly labeled using a conjugated antibody (e.g. Alexa Fluor® 488, Alexa Fluor® 647 and DyLight 350). Indirect detection relies on the use of an unconjugated primary antibody and a conjugated secondary antibody raised against the specie of the primary antibody. Due to signal amplification by secondary antibodies, indirect detection is often the preferred method for immunohistochemistry (IHC) staining. Detection can be performed through visualization of fluorescence (fluorescent labels) or chromogenic insoluble end products (enzyme labels). Both chromogenic and fluorescent detection methods have unique advantages and disadvantages and is contingent on your experimental needs as described below.
Chromogenic Detection vs. Fluorescent Detection
|
Advantages |
Disadvantages |
|
| Fluorescent Detection |
|
|
| Chromogenic Detection |
|
|
Fluorescent Detection
How does fluorescent detection work? Fluorescent detection requires a fluorochrome conjugated antibody to emit light when stimulated with a light of a shorter wavelength.
Can I use a conjugated primary antibody in my IF experiment? In the indirect method of detection, multiple secondary antibodies can bind to a single primary antibody. Because of its ability to amplify signal, indirect detection is often the method of choice for IHC/IF experiments. Staining tissue antigen with a primary conjugated antibody is only recommended for highly abundant tissue antigens where signal amplification is not necessary.
Can I run multiplex IHC using fluorescent detection? The large number of available fluorochromes allow the simultaneous detection of multiple targets due to their ability to emit light at unique wavelengths. Fluorochromes should be chosen carefully in multiplex experiments to minimize spectral overlap. In addition, multiplex fluorescent experiments should be designed to limit cross-reactivity. By choosing primary antibodies from different host species, difficulties concerning cross reactivity can largely be ignored. In this case, species-specific secondary antibodies will recognize only one primary antibody.
Example of IHC Detection by Immunofluorescence (IF):
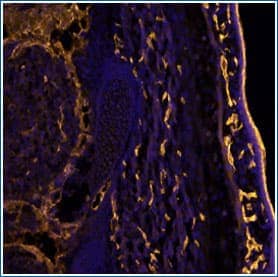
CD31/PECAM-1 in Mouse Embryo. CD31 staining using Goat Anti-Mouse CD31/PECAM 1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3628).
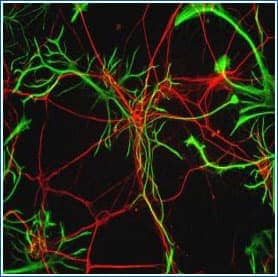
GFAP in Rat Neurons. GFAP (green) and Neurofilament Heavy (red) staining using Rabbit Anti-Rat GFAP Polyclonal Antibody (Catalog # NB300-141) and Chicken Anti-Rat NF-H Polyclonal Antibody (Catalog # NB300-217).
Chromogenic Detection
How does chromogenic detection work? Antigen expression is visualized in chromogenic detection when a soluble substrate is converted by an enzyme to an insoluble colored product that is deposited at the site of antigen expression. The enzymes horseradish peroxidase (HRP) and alkaline phosphatase are often used in chromogenic detection and function by converting 3,3' diaminobenzidine (DAB) and 3-amino-9-ethylcarbazole (AEC), into brown and red end products, respectively.
Should I use DAB, AEC, or a different chromogen? DAB is more popular than AEC due its longevity and resistance to fading when exposed to light. If multiplexing, it is recommended to choose chromogens with opposing colors to limit spectral overlap.
Can I run multiplex IHC using chromogenic detection? Although visualization of multiple antigens is possible by chromogenic detection, the deposition of two colors on co-localized proteins may obscure results. Therefore, it is recommended to multiplex only when the antigens are confined to unique cellular locations. This will allow easier differentiation of each target.
Example of Chromogenic IHC Detection:
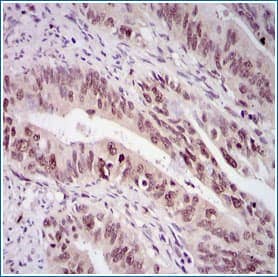
Survivin in Human Rectal Cancer. Survivin staining using Rabbit Anti-Human Survivin Antigen Affinity-purified Polyclonal Antibody (Catalog # NB500-201).
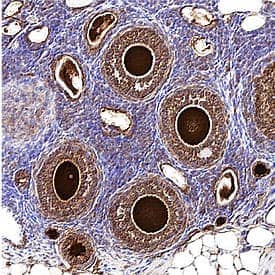
Stella/Dppa3 in Mouse Ovarian Tissue. Distinct staining distinguishes developing mouse oocytes from ovarian tissue using Goat Anti-Mouse Stella/Dppa3 polyclonal antibody (Catalog # AF2566).
Signal Amplification Using
Chromogenic Detection
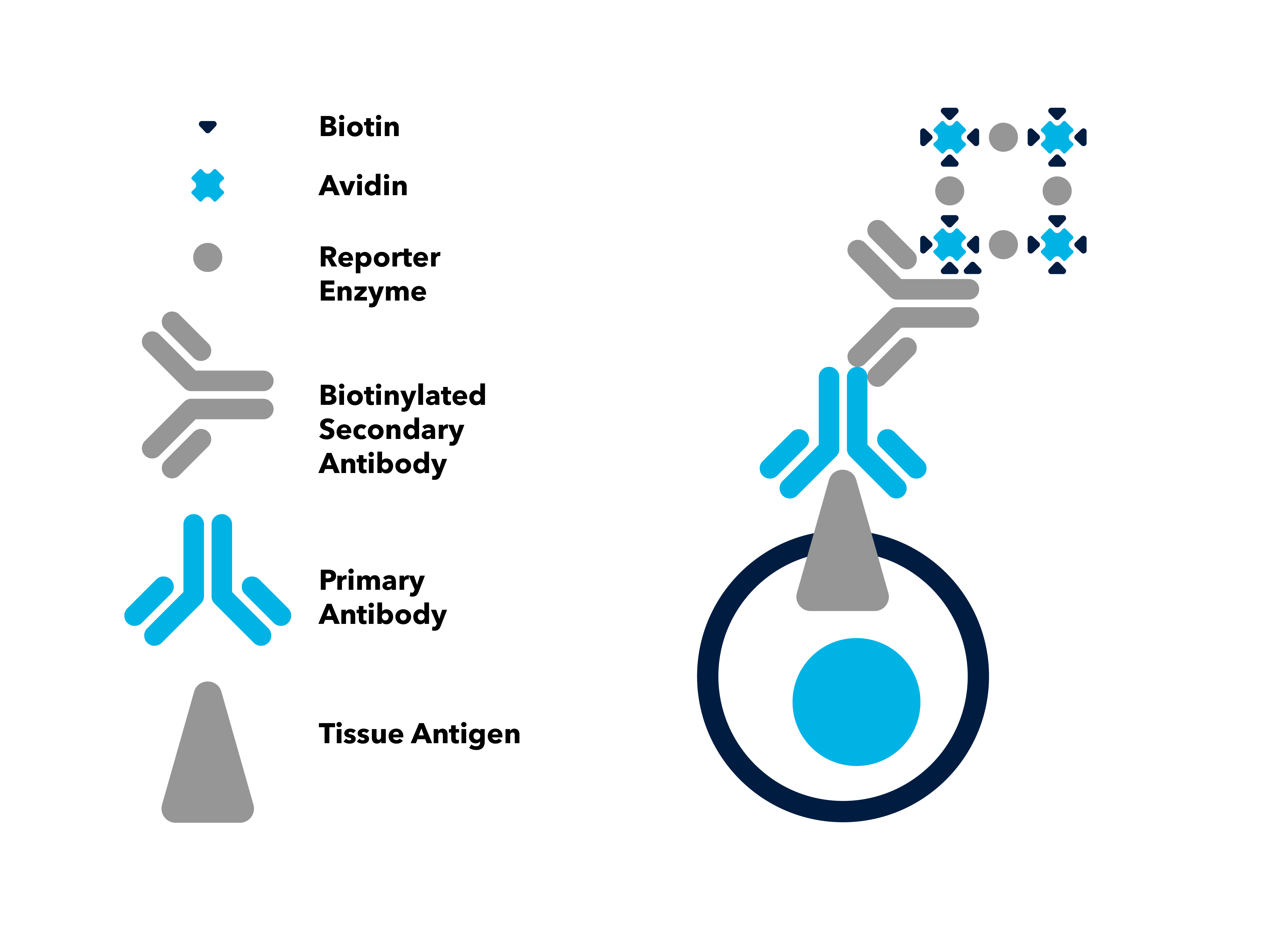
Avidin-Biotin Complex (ABC) Method
In the avidin-biotin complex (ABC) method, biotin conjugated secondary antibodies link tissue-bound primary antibodies with an avidin-biotin-peroxidase complex.The avidin molecule contains four binding sites for biotin. These binding sites enable complexes to form, where avidin molecules are linked together via the enzyme. A colorless substrate is then added and subsequently converted to a brown product by the peroxidase enzyme to mark the target antigen. The large complexes formed in the ABC method contain multiple copies of the reporter enzyme. Because of the high enzyme-to-antibody ratio, the ABC method increases sensitivity compared to direct conjugation of enzyme to the secondary antibody.
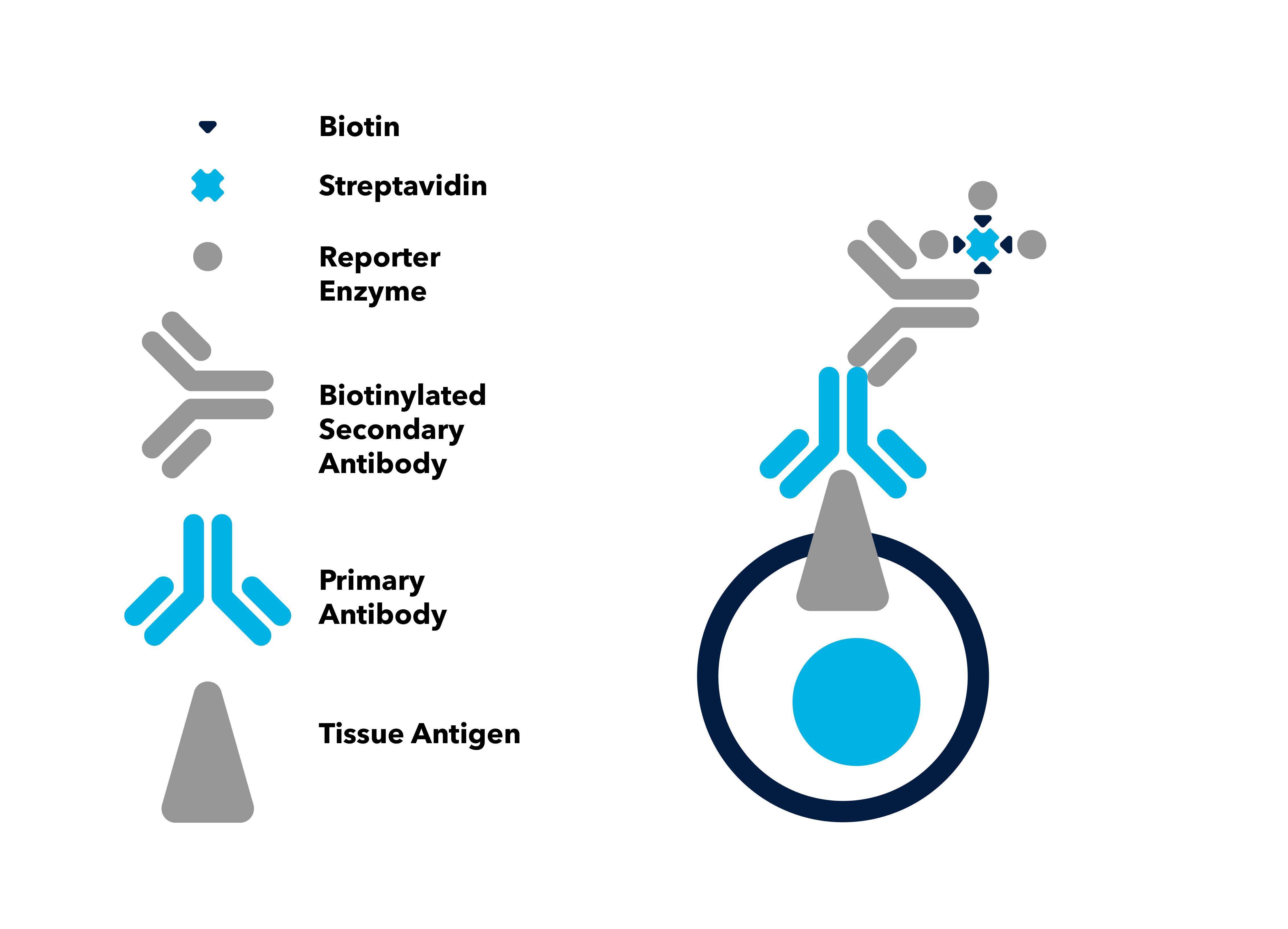
Labeled Streptavidin-Biotin (LSAB) Method
Similar to the avidin-biotin complex (ABC) method, the labeled streptavidin-biotin (LSAB) method uses a biotin conjugated secondary antibody to link the primary antibody to a streptavidin-peroxidase complex.The advantage of this approach is the lack of need for the ABC complex and the overall smaller complex size. The smaller complex, compared to the ABC method, facilitates tissue penetration and can enhance sensitivity.
Summary of Signal Amplification Methods
|
Complex Formed |
Advantages |
|
| ABC Method | Avidin-biotin-enzyme |
|
| LSAB Method | Streptavidin-enzyme |
|
| Polymer-based Method | Polymer backbone- enzyme |
|