By Victoria Osinski
Pyroptosis is an inflammatory form of programmed cell death characterized by the release of pro-inflammatory cytokines IL-1β and IL-18.1,10 It is a process distinct from apoptosis and necrosis (Table 1) and has been observed in the setting of multiple diseases including, but not limited to, microbial infection10, atherosclerosis and other cardiovascular diseases6, sepsis4, and liver disease5. Of note, cell death is defined as “programmed” when a cell relies on its own intrinsic cellular signaling to initiate the process.2 The canonical form of pyroptosis is defined as caspase-1-dependent, though multiple studies have identified caspase-1-independent pyroptosis pathways. Pyroptosis and its molecular mechanisms are a growing area of interest because they serve as potential novel therapeutic targets for a number of diseases.9
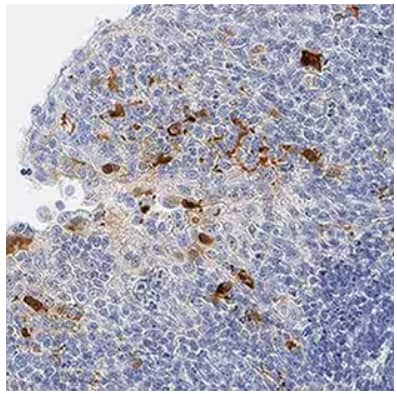
Immunohistochemical analysis from paraffin-embedded sections of human tonsil showing IL‑1 beta /IL‑1F2 detected by using Goat Anti-Human IL‑1 beta /IL‑1F2 Polyclonal Antibody (AF-201-NA) followed by incubation with IgG VisUCyte™ HRP Polymer Antibody (VC004). Tissue was then stained with DAB (brown) and counterstained using hematoxylin (blue). Specific staining was localized to cytoplasm in lymphocytes.
Table 1: Differences between apoptosis, necrosis, and pyroptosis.
| Apoptosis | Necrosis | Pyroptosis | |
|---|---|---|---|
| Programmed? | Yes | No | Yes |
| Membrane maintained? | Yes | No | No |
| Components of cellular process | Cytoplasmic and nuclear condensation, DNA damage, Formation of apoptotic bodies, Exposure of surface molecules that recruit phagocytosis | Organelle swelling, DNA damage (sometimes) | Caspase-1 activation, DNA damage, Cytokine release |
| Inflammatory cellular components release? | No | Yes | Yes |
| Caspases involved | Caspase-2, -3, -6, -7, -8, -9 (mouse), -10 (human) | Caspase-1, -3, -11 (mouse); -4, -5 (human) |
Based on findings summarized in References 2, 3, 10
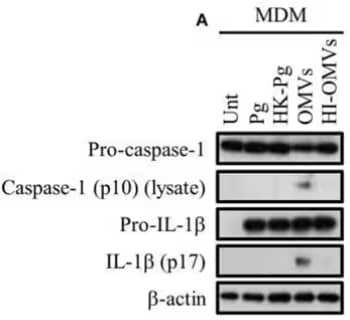
Western blot highlighting that P. gingivalis and its outer membrane vesicles (OMVs) differentially induce inflammasome signaling and pyroptosis in human macrophages. Monocyte-derived macrophages (MDM) were infected with viable P. gingivalis (Pg), heat-killed-Pg (HK-Pg), OMVs, or heat-inactivated-OMVs (HI-OMVs) and the activation of inflammasome components in the lysates was measured after 24 h by Western blot using Mouse Anti-Caspase-1 Monoclonal Antibody (NB100-56565) and Goat Anti-IL-1 beta Polyclonal Antibody (AF-401-NA); beta-actin serves as a loading control. Image collected and cropped by CiteAb from the following publication (//journal.frontiersin.org/article/10.3389/fcimb .2017.00351/full), licensed under a CC-BY license.
Caspase-1-dependent pyroptosis:
Caspase 1 is induced by the pattern recognition receptors Nucleotide-binding oligomerization domain and leucine-rich repeat receptors (NLRs), including NLRP3, NLRP1b, and NLRC4/CARD12.1 These receptors can recognize and respond to PAMPs and DAMPs originating from bacterial, viral, and host cell components. Following NLR-Caspase 1 activation, the inflammasome is formed. The inflammasome is a multi-protein structure formed by sensors (such as NLRP3), inflammatory caspases, and an inflammasome adaptor protein such as apoptosis-associated speck-like protein containing a carboxy-terminal CARD (ASC) (Table 2).8,9,10
In addition to forming the inflammasome, inflammatory Caspases-1, -4/5, and -11 also activate Gasdermin D, which forms pores in the plasma membrane to release pro-inflammatory cytokines.7,10 This component of pyroptosis, the active release of pro-inflammatory cytokines, is what make it particularly unique relative to other forms of cell death. Pro-inflammatory cytokines IL-1β and IL-18 are first translated into non-active forms, which are then cleaved and activated by Caspase-1.3,7,10 These cytokines can then be released via Gasdermin D-formed pores.
Caspase-1-independent pyroptosis:
Gasdermin D can also be activated by Caspase-4,-5, and -11 in a Caspase-1-independent form of pyroptosis. Though, it should be noted that activation of pro-inflammatory IL-1β and IL-18 still rely on Caspase-1 activity in this context.10 Caspase-3 has also been shown to activate Gasdermin E to form a pore for release of DAMPs and cytokines.3
New information concerning the mechanisms driving pyroptosis continues to emerge and it is evident that this is a complex problem. These advances in our understanding of pyroptosis also reveal its importance in human health and provide opportunities to identify new therapeutic targets.
Table 2: Inflammasome components
| Sensor molecule | Adaptor protein | Caspases |
|---|---|---|
| NLRP1, -3, -4, -6, -7, -12; NLRC4; AIM2; pyrin; IFI16; RIG-1 | ASC, Pycard | Caspase-1, -4/5*, -11* |
*Not necessary for inflammasome assembly, but can contribute
Based on findings summarized in References 1, 8, 9
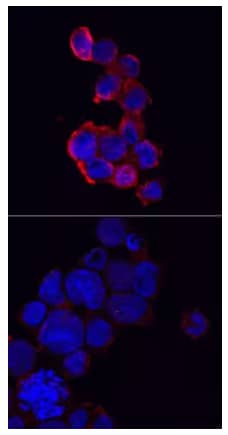
Immunocytochemical staining of immersion fixed THP‑1 human acute monocytic leukemia cells either treated with PMA and LPS (upper panel) or untreated (lower panel) and probed with Anti-NLRP3/NALP3 Polyclonal Antibody (AF6789), followed by staining with NorthernLights™ 557-conjugated Secondary Antibody (NL010) (red) and counterstained with DAPI (blue). Specific staining was localized to cytoplasm.

Victoria Osinski, Doctoral Candidate
University of Virginia
Victoria studies cellular mechanisms regulating vascular growth during peripheral artery disease and obesity.
-
Bergsbaken, T. et al. (2009) Pyroptosis: host cell death and inflammation Nature reviews. Microbiology
-
Fink, S. L., & Cookson, B. T. (2005) Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells Infection and immunity
-
Frank, D., & Vince, J. E. (2019) Pyroptosis versus necroptosis: similarities, differences, and crosstalk Cell death and differentiation
-
Gao, Y. L. et al. (2018) Recent Advances in the Molecular Mechanisms Underlying Pyroptosis in Sepsis Mediators of inflammation
-
Guo, H. et al. (2019) The relevance of pyroptosis in the pathogenesis of liver diseases Life sciences
-
Jia, C. et al. (2019) Role of pyroptosis in cardiovascular diseases International immunopharmacology
-
Kesavardhana, S., & Kanneganti, T. (2017) Mechanisms governing inflammasome activation, assembly and pyroptosis induction International immunology
-
Latz, E., Xiao, T. S., & Stutz, A. (2013) Inflammasome activation and assembly at a glance Nature reviews. Immunology
-
Man, S. M. et al. (2017) Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases Immunological reviews
-
Swanson, K. V. et al. (2019) The NLRP3 inflammasome: molecular activation and regulation to therapeutics Nature reviews. Immunology.