Human BMP-7 Antibody
R&D Systems, part of Bio-Techne | Catalog # MAB3541

Key Product Details
Validated by
Biological Validation
Species Reactivity
Validated:
Human
Cited:
Human, Mouse, Hamster
Applications
Validated:
Immunohistochemistry, Neutralization, Western Blot
Cited:
ELISA, ELISA Development, Immunocytochemistry, Immunohistochemistry, Immunohistochemistry-Paraffin, Immunoprecipitation, Neutralization, Western Blot
Label
Unconjugated
Antibody Source
Monoclonal Mouse IgG2B Clone # 164311
Product Specifications
Immunogen
Chinese hamster ovary cell line CHO-derived recombinant human BMP-7
Ser293-His431
Accession # P18075
Ser293-His431
Accession # P18075
Specificity
Detects human BMP-7 in direct ELISAs and Western blots. In direct ELISAs, approximately 25% cross-reactivity with recombinant human (rh) BMP-6 is observed and no cross-reactivity with rhBMP-2, -3, -4, -5, or -8 is observed.
Clonality
Monoclonal
Host
Mouse
Isotype
IgG2B
Endotoxin Level
<0.10 EU per 1 μg of the antibody by the LAL method.
Scientific Data Images for Human BMP-7 Antibody
Alkaline Phosphatase Production Induced by BMP‑7 and Neutralization by Human BMP‑7 Antibody.
Recombinant Human BMP-7 (Catalog # 354-BP) induces alkaline phosphatase production in the the ATDC5 mouse chondrogenic cell line in a dose-dependent manner (orange line). Alkaline phosphatase production elicited by Recombinant Human BMP-7 (1 µg/mL) is neutralized (green line) by increasing concentrations of Mouse Anti-Human BMP-7 Monoclonal Antibody (Catalog # MAB3541). The ND50 is typically 1.5-6.0 µg/mL in the presence of L-ascorbic acid (50 µg/mL).BMP‑7 in Human Kidney.
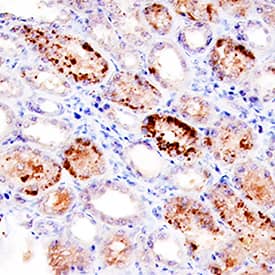
BMP-7 was detected in immersion fixed paraffin-embedded sections of human kidney using Mouse Anti-Human BMP-7 Monoclonal Antibody (Catalog # MAB3541) at 15 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Mouse HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS002) and counterstained with hematoxylin (blue). Specific staining was localized to cytoplasm in convoluted tubules. View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.Detection of Human BMP-7 by Immunohistochemistry
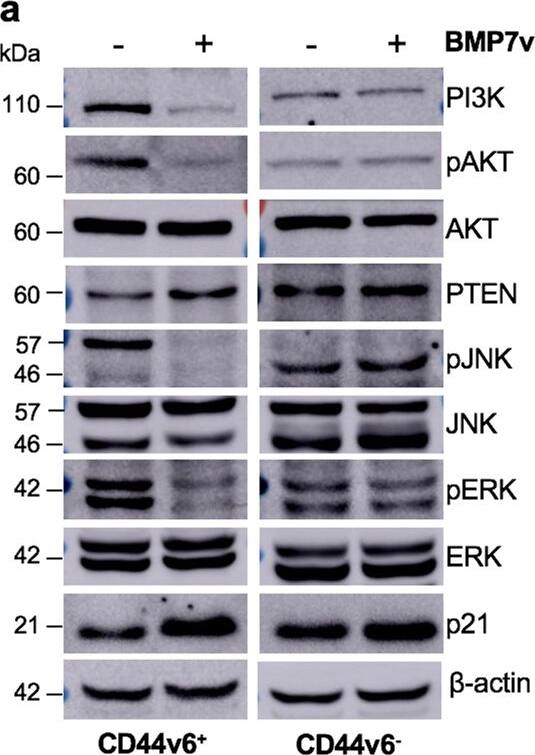
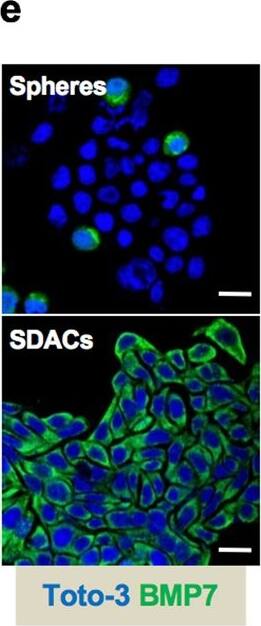
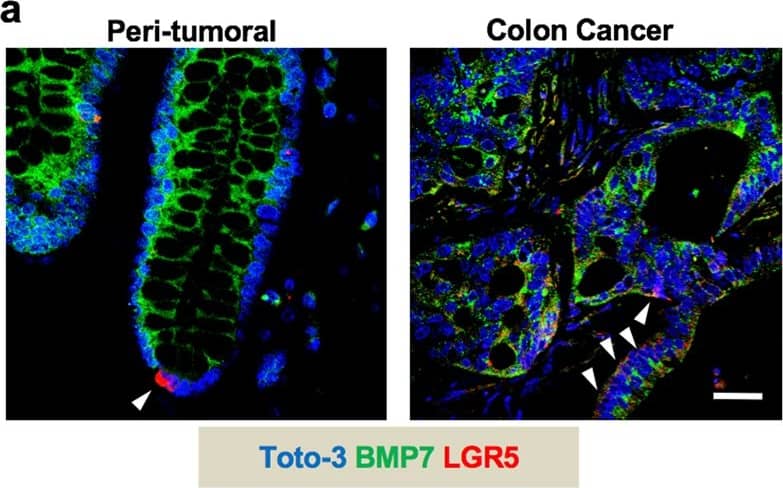
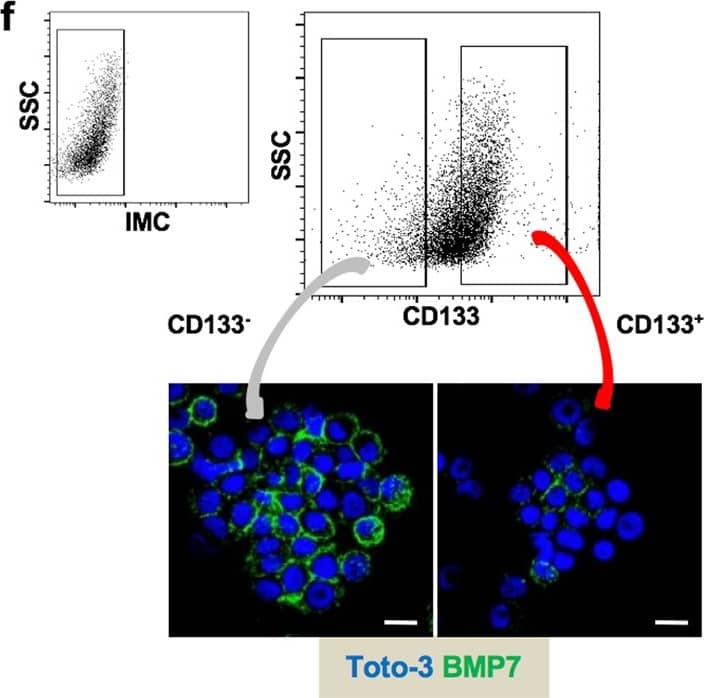
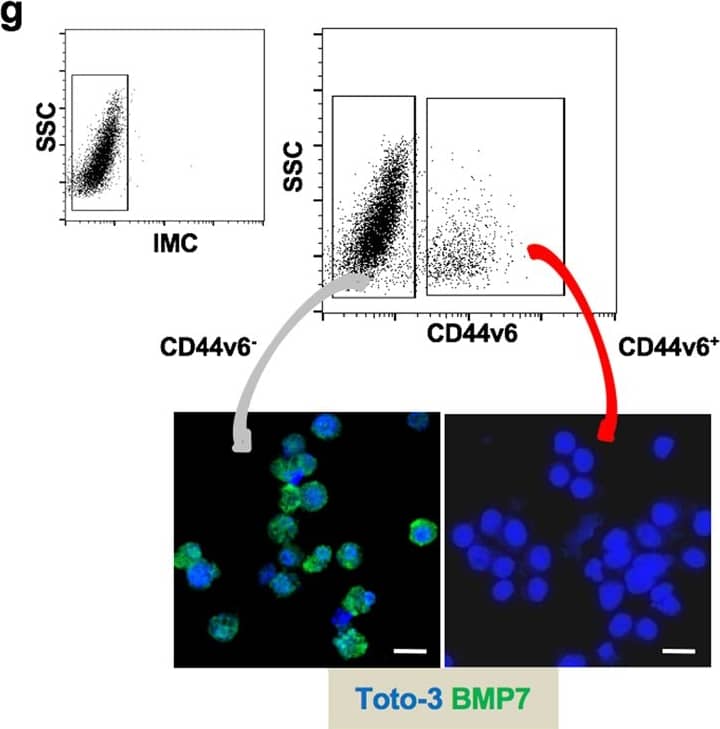
BMP7v exerts antiangiogenic effects and sensitizes chemoresistant CSCs to standard therapy. a Azan-Mallory staining on paraffin-embedded sections of xenografts derived from the injection of CRC sphere cells and treated for 4 weeks (6–9 weeks) with PBS (vehicle) or BMP7v. Data are representative of three independent experiments using different CRC sphere cell lines (CSC#2, 7, and 18). b Percentage of necrosis evaluated on paraffin-embedded sections of xenografts treated as in a. Data are shown as mean ± SD of three independent experiments. c Immunohistochemical analysis of CD31 and VEGFR2 (red staining) on paraffin-embedded sections of xenografts generated by the injection of CRC sphere cell lines and treated with PBS (vehicle), BMP4, or BMP7v. Green arrowheads indicate microvessels expressing CD31 or VEGFR2. Images are representative of three independent experiments using cells as in a. Nuclei were revealed by hematoxylin staining (blue). The scale bar represents 20 µm. d Number of microvessels positive for CD31 (left panel) and VEGFR2 (right panel) expression, evaluated on paraffin-embedded sections of xenografts treated as in c. Data are shown as mean ± SD of cells. MVD = microvessel density. e Fold change of viable cells in 35 CR-CSC lines treated with oxaliplatin/5-FU for 24 h. Dotted line indicates the threshold between chemoresistant (red) and sensitive CR-CSCs (green). f Cell viability percentage in chemoresistant CR-CSCs (R1-R4) pretreated with BMP7 for 3 days and with oxaliplatin/5-FU (oxa/5-FU) for additional 24 h as indicated. Data are shown as mean ± SD of three different experiments performed in the indicated R-CSCs. g Colony forming efficiency of CR-CSCs treated as in f and evaluated at 21 days. Representative soft-agar analyses are reported in the lower part of the graph. Bars show the mean ± SD of seven different CRC sphere cell lines (CSC#1–3, 5, 7, 10, and 18). h Tumor size of subcutaneous growth of the indicated CR-CSCs. Mice were treated for 4 weeks (6–9 weeks) with vehicle, oxaliplatin/5-FU (oxa/5-FU) and BMP7v alone or in combination. Error bars show the mean ± SD of tumor size measured in six mice/group. Black arrowheads indicate days of treatment. i Immunohistochemical analysis of CD44v6, beta-catenin, Ki67, and CK20 (red color) in paraffin-embedded sections of CSC#7 xenografts treated as in h. Nuclei were counterstained by aqueous hematoxylin (blue color). The scale bar represents 20 µm (left panels). Percentage of CD44v6, beta-catenin, Ki67, and CK20 positive cells in paraffin-embedded sections of tumor xenografts treated with vehicle (V), BMP7v (B), oxaliplatin/5-FU (O/F), alone or in combination (B/O/F) for 72 h. Error bars are mean ± SD of positive cell counts in three serial embedded-paraffin sections of six tumor xenografts per group derived from the injection of three different CRC sphere cells (CSC#1, 2, and 7) (right panels) Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31591478), licensed under a CC-BY license. Not internally tested by R&D Systems.Applications for Human BMP-7 Antibody
Application
Recommended Usage
Immunohistochemistry
8-25 µg/mL
Sample: Immersion fixed paraffin-embedded sections of human kidney tissue
Sample: Immersion fixed paraffin-embedded sections of human kidney tissue
Western Blot
1 µg/mL
Sample: Recombinant Human BMP‑7 (Catalog # 354-BP) under non-reducing conditions only
Sample: Recombinant Human BMP‑7 (Catalog # 354-BP) under non-reducing conditions only
Neutralization
Measured by its ability to neutralize BMP-7-induced alkaline phosphatase production in the ATDC5 mouse chondrogenic cell line. Erlacher, L. et al. (1998) J. Bone Miner. Res. 13:383. The Neutralization Dose (ND50) is typically 1.5-6.0 µg/mL in the presence of 1 µg/mL Recombinant Human BMP-7 and 50 µg/mL L-ascorbic acid.
Formulation, Preparation, and Storage
Purification
Protein A or G purified from hybridoma culture supernatant
Reconstitution
For liquid material, refer to CoA for concentration.
Formulation
Supplied as a 0.2 μm filtered solution in PBS with Trehalose. *Small pack size (SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Shipping
Lyophilized product is shipped at ambient temperature. Liquid small pack size (-SP) is shipped with polar packs. Upon receipt, store immediately at the temperature recommended below.
Stability & Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
- 12 months from date of receipt, -20 to -70 °C, as supplied.
- 1 month, 2 to 8 °C under sterile conditions after opening.
- 6 months, -20 to -70 °C under sterile conditions after opening.
Background: BMP-7
References
- Chen, D. et al. (2004) Growth Factors 22:233.
- Kishigami, S. and Y. Mishina (2005) Cytokine Growth Factor Rev. 16:265.
- Ozkaynak, E. et al. (1990) EMBO J. 9:2085.
- Celeste, A.J. et al. (1990) Proc. Natl. Acad. Sci. 87:9843.
- Gregory, K.E. et al. (2005) J. Biol. Chem. 280:27970.
- Sengle, G. et al. (2008) J. Mol. Biol. 381:1025.
- Israel, D.I. et al. (1996) Growth Factors 13:291.
- Aono, A. et al. (1995) Biochem. Biophys. Res. Commun. 210:670.
- Nishimatsu, S. and G.H. Thomsen (1998) Mech. Dev. 74:75.
- Sampath, T.K. et al. (1992) J. Biol. Chem. 267:20352.
- Kazama, I. et al. (2008) J. Am. Soc. Nephrol. 19:2181.
- Grishina, I.B. et al. (2005) Dev. Biol. 288:334.
- Zeisberg, M. et al. (2003) Nat. Med. 9:964.
- Buijs, J.T. et al. (2007) Am. J. Pathol. 171:1047.
- Yu, M.-A. et al. (2009) J. Am. Soc. Nephrol. 20:567.
- Chou, J. et al. (2006) J. Neurol. Sci. 240:21.
Long Name
Bone Morphogenetic Protein 7
Alternate Names
BMP7, OP-1
Gene Symbol
BMP7
UniProt
Additional BMP-7 Products
Product Documents for Human BMP-7 Antibody
Product Specific Notices for Human BMP-7 Antibody
For research use only
Loading...
Loading...
Loading...
Loading...