Detection of Human IL-13R alpha 2 by Flow Cytometry
Evaluation of additional commercially available B-D13 antibodies.(A) PBT008 cells that had been cultured overnight in media alone (black histogram) versus cytokine (TNF; red histogram) conditions, (B) Parental 293T cells or 293T cells engineered to express either VCAM-1 or IL13R alpha2, and (C) U251T cells were stained with VCAM-1-PE, AF146 or various B-D13 reagents – two lots of PE-conjugated B-D13 antibody (B-D13-PE; Cell Sciences) and two to three unconjugated B-D13 antibodies (B-D13-unc) purchased from either Cell Sciences (Cell Sci), Abcam or Santa Cruz as indicated. (B, C) Black histograms represent staining with istoype control antibody or SA-PE alone. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pone.0095123), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human IL-13R alpha 2 by Western Blot
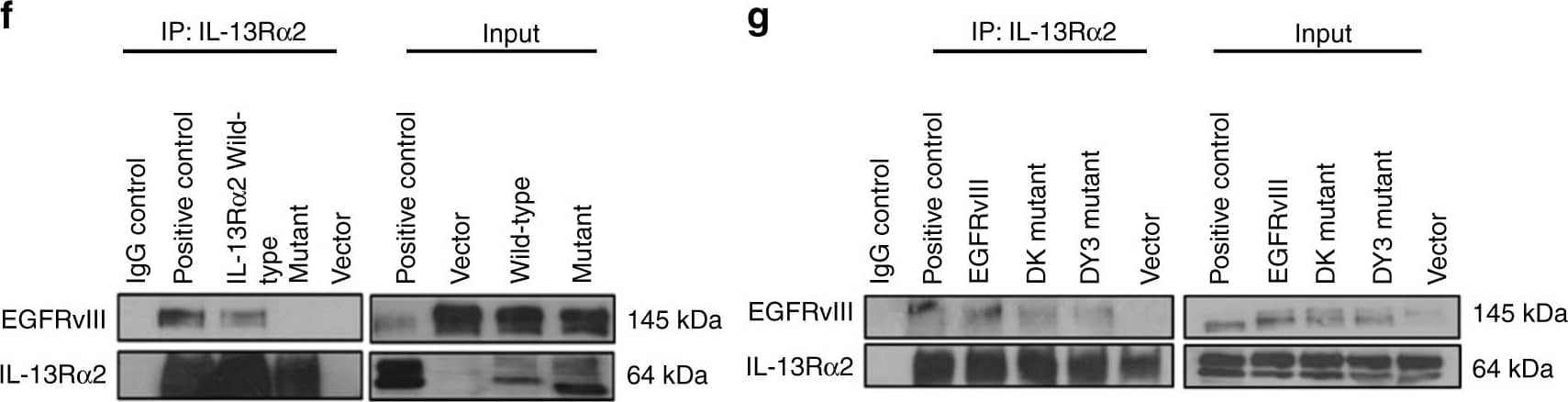
Deletion of the cytoplasmic domain of IL-13R alpha2 resulted in a loss of physical interaction with EGFRvIII and enhanced proliferation is abolished. a Whole-cell lysates prepared from stable cell line Gli36.IL-13R alpha2/EGFRvIII cells were used for immunoprecipitation with anti-IL-13R alpha2 antibody, then immunoprobed with an anti-EGFR antibody. IgG served as control while unprecipitated extracts serve as input. b Similar cell lysates were reverse immunoprecipitated with anti-EGFR antibody, then immunoprobed with an anti-IL13R alpha2antibody. Lysates from Gli36.EGFRvIII served as additional control c Gli36.IL-13R alpha2/EGFRvIII cell lysates were immunoprecipitated with anti-EGFR antibody, then immunoprobed with anti-Grb antibody. To further examine the domains of interaction, IL-13R alpha2 and EGFR mutants were used. Gli36.EGFRvIII cells were first transfected with pIRESneo2 (Vector), IL-13R alpha2 full length (Wild-type) and IL-13R alpha2 Cyt tail deleted constructs (Mutant) and then analyzed by d cell proliferation assay at the indicated time points, f co-immunoprecipitation, and h PLA assays. Findings were validated using Gli36.IL-13R alpha2 cells transiently transfected with vector (CTRL), full length/wild-type EGFRvIII, DK, and DY3 mutants. e proliferation outputs, g co-immunoprecipitation, i and PLA assay were performed. j represent the corresponding positive and negative controls. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/29203859), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human IL-13R alpha 2 by Western Blot
GBM patients co-expressing EGFR and IL-13R alpha2 correlate to poor survival where the overexpression of IL-13R alpha2 alone leads to enhance cell migration but not proliferation. Kaplan−Meier survival analysis of a all gliomas patients; b GBM patients from REMBRANDT database from National Cancer Institute (USA). Patients overexpressing EGFR mRNA by 2-fold (blue) with high (red), intermediate (yellow) and low (green) levels of IL-13R alpha2 expression were shown. The log-rank p-values were indicated. c Kaplan−Meier survival plots for patients expressing high YKL-40 mRNA levels TCGA. High IL-13R alpha2 expression group (red) and low IL-13R alpha2 expression group (blue) were determined by aggregating all patients whose z-score normalized expression was above or below 0, respectively (Log-rank test p-value = 0.0374). Immunoblotting analysis showed the expression of EGFR and IL-13R alpha2 protein levels were determined from d a panel of 10 patient-derived GBM e and the isogenic cell lines generated from Gli36 glioma cells. Pan-actin or beta tubulin served as internal loading controls. f Cell proliferation and g Cell cycle analysis were performed with Gli36 and Gli36.IL-13R alpha2 cells h Soft agar colony formation assay was performed, Gli36.EGFRvIII was used as a positive control. i In vitro migration and j invasion assays were determined in Gli36 and Gli36.IL-13R alpha2 cells. All data are represented as mean ± SEM, unpaired t-test **p < 0.01; ***p < 0.001; NS not significant. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/29203859), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human IL-13R alpha 2 by Flow Cytometry
The cytokine-induced cell surface antigen recognized by B-D13-PE does not bind IL-13.(A) D283 cells engineered to express IL13R alpha2 (D283- IL13R alpha2) (blue histograms) and D283 parental (black histograms) cells were evaluated by flow cytometry for expression of constitutive IL13R alpha2 using the IL13R alpha2-specific antibody AF146, and biotinylated recombinant human IL-13 (IL13-bio) followed by PE-conjugated strepavidin (SA-PE). Data are representative of 2 separate experiments. (B) U251T (grown in the absence of cytokines) were evaluated by flow cytometry for constitutive IL13R alpha2 expression using AF146, and for binding to IL13-bio/SA-PE in the presence and absence of 10-fold molar excess of recombinant human IL-4 or IL-13. Black histograms represent staining with istoype control antibody or SA-PE alone. Data are representative of 2 separate experiments. (C) THP-1 and PBT008 grown in media alone (black histograms) or induced overnight with TNF and IL-4 (red histograms) were analyzed by flow cytometry for expression of constitutive IL13R alpha2 (AF146), for expression of the induced antigen (B-D13-PE), and for binding to IL13-bio/SA-PE. Data are representative of 3 separate experiments. (D) IL13-zetakine+ CD8+ CTL recognize and kill U251T glioma targets expressing constitutive IL13R alpha2 (AF146-positive), but not cytokine-induced PBT003 cells (B-D13-positive). Percentage specific lysis (mean ± S.D.) of triplicate wells is depicted. *, p≤0.0002 using an unpaired Student's t-test to compare U251T vs. PBT003-4 targets. #, p>0.05 using an unpaired Student's t-test to compare PBT003-4 targets with and without overnight cytokine stimulation. Data are representative of at least 2 separate experiments. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pone.0095123), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human IL-13R alpha 2 by Flow Cytometry
Evaluation of additional commercially available B-D13 antibodies.(A) PBT008 cells that had been cultured overnight in media alone (black histogram) versus cytokine (TNF; red histogram) conditions, (B) Parental 293T cells or 293T cells engineered to express either VCAM-1 or IL13R alpha2, and (C) U251T cells were stained with VCAM-1-PE, AF146 or various B-D13 reagents – two lots of PE-conjugated B-D13 antibody (B-D13-PE; Cell Sciences) and two to three unconjugated B-D13 antibodies (B-D13-unc) purchased from either Cell Sciences (Cell Sci), Abcam or Santa Cruz as indicated. (B, C) Black histograms represent staining with istoype control antibody or SA-PE alone. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pone.0095123), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse IL-13R alpha 2 by Flow Cytometry
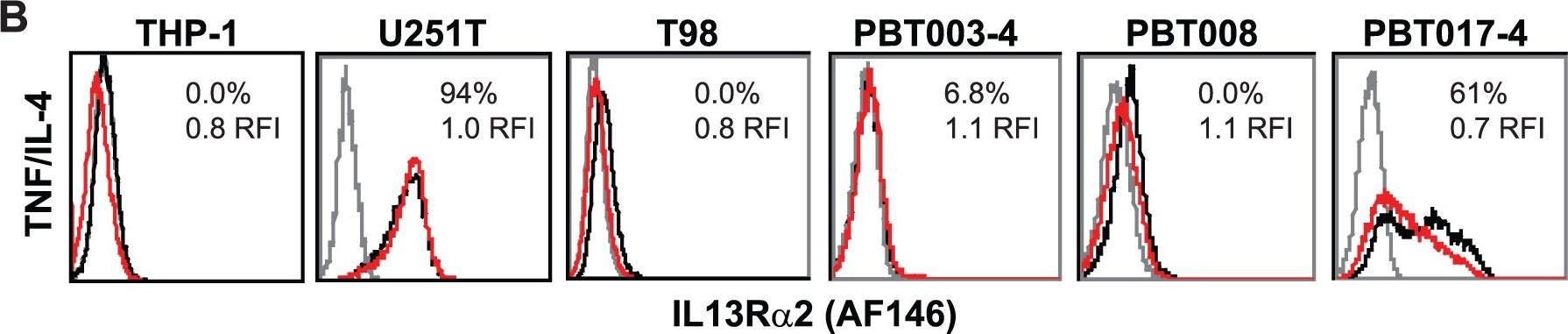
Differential recognition of constitutively-expressed versus cytokine-induced IL13R alpha2 by commercially available anti-IL13R alpha2 antibodies.Flow cytometric analysis of monocytic line THP-1 and various glioma lines with (A) B-D13-PE (Cell Sciences) or (B) AF146 (R&D Systems) reagents for media alone (black histogram) and cytokine (TNF/IL-4 or TNF/IL-13 overnight; red histogram) conditions. Isotype (iso-PE) and mouse anti-goat-FITC controls are shown as grey histograms. Percent positive and relative fluorescent index (RFI) of MFI cytokine/MFI media is reported for each histogram. (C) Flow cytometric detection of IL13R alpha2 for D283 cells engineered to express IL13R alpha2 (D283-IL13R alpha2; blue histogram) and D283 parental (black histogram) stained with AF146 or B-D13-PE antibodies. All data are representative of more than three experiments each. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pone.0095123), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human IL-13R alpha 2 by Western Blot
Deletion of the cytoplasmic domain of IL-13R alpha2 resulted in a loss of physical interaction with EGFRvIII and enhanced proliferation is abolished. a Whole-cell lysates prepared from stable cell line Gli36.IL-13R alpha2/EGFRvIII cells were used for immunoprecipitation with anti-IL-13R alpha2 antibody, then immunoprobed with an anti-EGFR antibody. IgG served as control while unprecipitated extracts serve as input. b Similar cell lysates were reverse immunoprecipitated with anti-EGFR antibody, then immunoprobed with an anti-IL13R alpha2antibody. Lysates from Gli36.EGFRvIII served as additional control c Gli36.IL-13R alpha2/EGFRvIII cell lysates were immunoprecipitated with anti-EGFR antibody, then immunoprobed with anti-Grb antibody. To further examine the domains of interaction, IL-13R alpha2 and EGFR mutants were used. Gli36.EGFRvIII cells were first transfected with pIRESneo2 (Vector), IL-13R alpha2 full length (Wild-type) and IL-13R alpha2 Cyt tail deleted constructs (Mutant) and then analyzed by d cell proliferation assay at the indicated time points, f co-immunoprecipitation, and h PLA assays. Findings were validated using Gli36.IL-13R alpha2 cells transiently transfected with vector (CTRL), full length/wild-type EGFRvIII, DK, and DY3 mutants. e proliferation outputs, g co-immunoprecipitation, i and PLA assay were performed. j represent the corresponding positive and negative controls. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/29203859), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human IL-13R alpha 2 by Western Blot
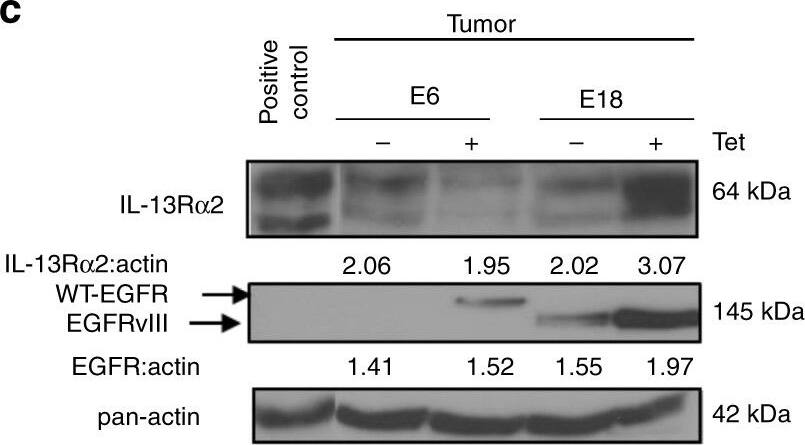
IL-13R alpha2 mediate greater tumorigenic potential with EGFRvIII, and not WT EGFR. a Tumor volume b and tumor weight of tetracycline regulatable U251 gliomas (U251-E6 and U251-E18 was examined in vivo. Bars depict the mean values and error bars represent 95% confidence intervals. P-values were calculated using ANOVA with Tukey’s multiple comparison test *p < 0.05; **p < 0.01; ***p < 0.001. Photomicrographs of represented collected tumors are shown. c Immunoblot analysis of proteins from U251-E6 and -E18 tumor lysates in the presence or absence of tetracycline with the indicated antibodies. One representative tumor under each of the uninduced and induced conditions was shown. U251MG whole-cell lysate served as positive control for IL-13R alpha2. d Kaplan−Meier survival curves of mice bearing U251-E6 and U251-18 tumors **p < 0.0039. Kaplan−Meier survival plots for patients expressing e high EGFR mRNA levels (excluding EGFRvIII) or f high EGFRvIII mRNA levels from TCGA database. High IL-13R alpha2 expression (red) and low IL-13R alpha2 expression (blue) were determined by aggregating all patients whose z-score normalized expression was above or below 0, respectively. g Schematic model showing signal transduction pathway co-induced by IL-13R alpha2 and EGFRvIII. Overexpression of IL-13R alpha2 in human gliomas increases cell migration and invasion through the activation of MMP-2, vimentin. Amplification of EGFRvIII promotes the co-interaction of both receptors mediating an increase in tyrosine kinase activities and a preferential activation of RAS-MEK-ERK and STAT3 pathways leading to aberrant cellular proliferation. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/29203859), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse IL-13R alpha 2 by Flow Cytometry
Cytokine induction of B-D13 antigen is dependent on mRNA transcription and translation.(A) IL13R alpha2 mRNA levels quantified by qPCR for U251T, PBT003-4, PBT008, and THP-1 cells after overnight culture in media, TNF/IL-4, or TNF/IL-13. mRNA levels were normalized to housekeeping genes (ACTB, UBC, GAPDH and RPLP0). (B, C) B-D13-PE immunoreactivity of PBT008 and THP-1 cells treated with increasing concentrations of (B) transcription blocker Actinomycin D (ActD) (0, 0.06, 0.1, 0.3 µg/ml) or (C) translation blocker Cycloheximide (CHX) (0, 2.5, 5, 10 µg/ml), then either cultured in media alone (black histograms) or stimulated with TNF/IL-4 (red histograms) for 5 hours. All data are representative of 2 separate experiments. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pone.0095123), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human IL-13R alpha 2 by Flow Cytometry
The cytokine-induced cell surface antigen recognized by B-D13-PE does not bind IL-13.(A) D283 cells engineered to express IL13R alpha2 (D283- IL13R alpha2) (blue histograms) and D283 parental (black histograms) cells were evaluated by flow cytometry for expression of constitutive IL13R alpha2 using the IL13R alpha2-specific antibody AF146, and biotinylated recombinant human IL-13 (IL13-bio) followed by PE-conjugated strepavidin (SA-PE). Data are representative of 2 separate experiments. (B) U251T (grown in the absence of cytokines) were evaluated by flow cytometry for constitutive IL13R alpha2 expression using AF146, and for binding to IL13-bio/SA-PE in the presence and absence of 10-fold molar excess of recombinant human IL-4 or IL-13. Black histograms represent staining with istoype control antibody or SA-PE alone. Data are representative of 2 separate experiments. (C) THP-1 and PBT008 grown in media alone (black histograms) or induced overnight with TNF and IL-4 (red histograms) were analyzed by flow cytometry for expression of constitutive IL13R alpha2 (AF146), for expression of the induced antigen (B-D13-PE), and for binding to IL13-bio/SA-PE. Data are representative of 3 separate experiments. (D) IL13-zetakine+ CD8+ CTL recognize and kill U251T glioma targets expressing constitutive IL13R alpha2 (AF146-positive), but not cytokine-induced PBT003 cells (B-D13-positive). Percentage specific lysis (mean ± S.D.) of triplicate wells is depicted. *, p≤0.0002 using an unpaired Student's t-test to compare U251T vs. PBT003-4 targets. #, p>0.05 using an unpaired Student's t-test to compare PBT003-4 targets with and without overnight cytokine stimulation. Data are representative of at least 2 separate experiments. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pone.0095123), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human IL-13R alpha 2 by Flow Cytometry
The cytokine-induced cell surface antigen recognized by B-D13-PE does not bind IL-13.(A) D283 cells engineered to express IL13R alpha2 (D283- IL13R alpha2) (blue histograms) and D283 parental (black histograms) cells were evaluated by flow cytometry for expression of constitutive IL13R alpha2 using the IL13R alpha2-specific antibody AF146, and biotinylated recombinant human IL-13 (IL13-bio) followed by PE-conjugated strepavidin (SA-PE). Data are representative of 2 separate experiments. (B) U251T (grown in the absence of cytokines) were evaluated by flow cytometry for constitutive IL13R alpha2 expression using AF146, and for binding to IL13-bio/SA-PE in the presence and absence of 10-fold molar excess of recombinant human IL-4 or IL-13. Black histograms represent staining with istoype control antibody or SA-PE alone. Data are representative of 2 separate experiments. (C) THP-1 and PBT008 grown in media alone (black histograms) or induced overnight with TNF and IL-4 (red histograms) were analyzed by flow cytometry for expression of constitutive IL13R alpha2 (AF146), for expression of the induced antigen (B-D13-PE), and for binding to IL13-bio/SA-PE. Data are representative of 3 separate experiments. (D) IL13-zetakine+ CD8+ CTL recognize and kill U251T glioma targets expressing constitutive IL13R alpha2 (AF146-positive), but not cytokine-induced PBT003 cells (B-D13-positive). Percentage specific lysis (mean ± S.D.) of triplicate wells is depicted. *, p≤0.0002 using an unpaired Student's t-test to compare U251T vs. PBT003-4 targets. #, p>0.05 using an unpaired Student's t-test to compare PBT003-4 targets with and without overnight cytokine stimulation. Data are representative of at least 2 separate experiments. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pone.0095123), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human IL-13R alpha 2 by Western Blot
Enhanced cellular proliferation mediated by IL-13R alpha2 is specific to EGFRvIII, and not WT EGFR. a U251-E6 or c U251-E18 cells were treated with or without tetracycline (Tet). At indicated time points, immunoblot analysis was carried out. Gli36, Gli36.EGFRvIII cell lysates were included as negative or positive controls for EGFRvIII, respectively. Growth kinetics of b U251-E6 and d U251-E18 was determined by CCK-8 assay. Percent cell viability was normalized to day 1 (without induction). All data are represented as mean ± SEM. Unpaired t-test ***p < 0.001, NS. not significant. e Co-immunoprecipitation was performed in stable cell lines Gli36.IL-13R alpha2/wtEGFR as well as U251MG-E6 (i.e. wtEGFR) cells at 48 h post tetracycline induction with the indicated antibodies. Gli36.IL-13R alpha2/EGFRvIII served as positive controls. f The interaction between endogenous wtEGFR and IL-13R alpha2 was shown in primary wtEGFR-positive GBM patient tumor derived from Mayo clinic, and IgG served as positive and negative controls respectively. Knockdown of IL-13R alpha2 in cell line or patient-derived GBM samples expressing g wtEGFR or h EGFRvIII. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/29203859), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human IL-13R alpha 2 by Flow Cytometry
B-D13 reagent appears to contain two distinct monoclonal antibodies.Soluble receptor competition study evaluating the specificity of IL13R alpha2 and VCAM-1 recognition by the B-D13-unc antibodies (Cell Sciences and Santa Cruz) using (A) PBT008 cells cultured overnight in media alone (black histogram) versus cytokine (TNF; blue histogram); or (B) IL13R alpha2-expressing U251T cells (blue histogram). Cells were stained with the indicated antibody that was pre-incubated with soluble recombinant human IL13R alpha-Fc (purple histograms) or VCAM-1-Fc (green histograms). Relative fluorescence index (RFI) compared to staining without the soluble competitors (i.e., the control/blue histograms) are indicated in each histogram. (C) Unconjugated B-D13 antibodies from Cell Sciences (top) and Abcam (bottom) were reduced and analyzed by LC/MS. Shown is the spectra of the deconvoluted protein masses depicting two distinct mass species for both the heavy and light chains. (D) Extracted ion chromatograms (EIC) for the two light chain species, of the Cell Sciences B-D13-unc reagent from (C). Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pone.0095123), licensed under a CC-BY license. Not internally tested by R&D Systems.
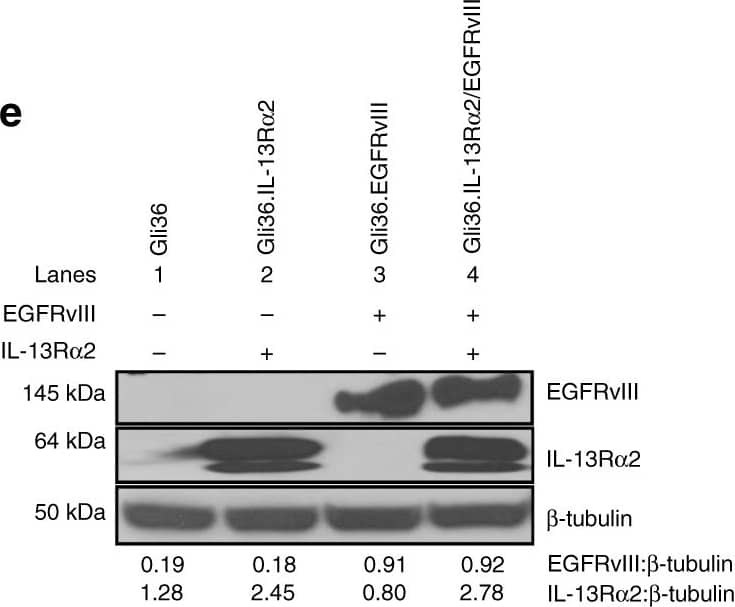
Detection of Human IL-13R alpha 2 by Western Blot
GBM patients co-expressing EGFR and IL-13R alpha2 correlate to poor survival where the overexpression of IL-13R alpha2 alone leads to enhance cell migration but not proliferation. Kaplan−Meier survival analysis of a all gliomas patients; b GBM patients from REMBRANDT database from National Cancer Institute (USA). Patients overexpressing EGFR mRNA by 2-fold (blue) with high (red), intermediate (yellow) and low (green) levels of IL-13R alpha2 expression were shown. The log-rank p-values were indicated. c Kaplan−Meier survival plots for patients expressing high YKL-40 mRNA levels TCGA. High IL-13R alpha2 expression group (red) and low IL-13R alpha2 expression group (blue) were determined by aggregating all patients whose z-score normalized expression was above or below 0, respectively (Log-rank test p-value = 0.0374). Immunoblotting analysis showed the expression of EGFR and IL-13R alpha2 protein levels were determined from d a panel of 10 patient-derived GBM e and the isogenic cell lines generated from Gli36 glioma cells. Pan-actin or beta tubulin served as internal loading controls. f Cell proliferation and g Cell cycle analysis were performed with Gli36 and Gli36.IL-13R alpha2 cells h Soft agar colony formation assay was performed, Gli36.EGFRvIII was used as a positive control. i In vitro migration and j invasion assays were determined in Gli36 and Gli36.IL-13R alpha2 cells. All data are represented as mean ± SEM, unpaired t-test **p < 0.01; ***p < 0.001; NS not significant. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/29203859), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human IL-13R alpha 2 by Flow Cytometry
The cytokine-induced cell surface antigen recognized by B-D13-PE does not bind IL-13.(A) D283 cells engineered to express IL13R alpha2 (D283- IL13R alpha2) (blue histograms) and D283 parental (black histograms) cells were evaluated by flow cytometry for expression of constitutive IL13R alpha2 using the IL13R alpha2-specific antibody AF146, and biotinylated recombinant human IL-13 (IL13-bio) followed by PE-conjugated strepavidin (SA-PE). Data are representative of 2 separate experiments. (B) U251T (grown in the absence of cytokines) were evaluated by flow cytometry for constitutive IL13R alpha2 expression using AF146, and for binding to IL13-bio/SA-PE in the presence and absence of 10-fold molar excess of recombinant human IL-4 or IL-13. Black histograms represent staining with istoype control antibody or SA-PE alone. Data are representative of 2 separate experiments. (C) THP-1 and PBT008 grown in media alone (black histograms) or induced overnight with TNF and IL-4 (red histograms) were analyzed by flow cytometry for expression of constitutive IL13R alpha2 (AF146), for expression of the induced antigen (B-D13-PE), and for binding to IL13-bio/SA-PE. Data are representative of 3 separate experiments. (D) IL13-zetakine+ CD8+ CTL recognize and kill U251T glioma targets expressing constitutive IL13R alpha2 (AF146-positive), but not cytokine-induced PBT003 cells (B-D13-positive). Percentage specific lysis (mean ± S.D.) of triplicate wells is depicted. *, p≤0.0002 using an unpaired Student's t-test to compare U251T vs. PBT003-4 targets. #, p>0.05 using an unpaired Student's t-test to compare PBT003-4 targets with and without overnight cytokine stimulation. Data are representative of at least 2 separate experiments. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pone.0095123), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse IL-13R alpha 2 by Flow Cytometry
Differential recognition of constitutively-expressed versus cytokine-induced IL13R alpha2 by commercially available anti-IL13R alpha2 antibodies.Flow cytometric analysis of monocytic line THP-1 and various glioma lines with (A) B-D13-PE (Cell Sciences) or (B) AF146 (R&D Systems) reagents for media alone (black histogram) and cytokine (TNF/IL-4 or TNF/IL-13 overnight; red histogram) conditions. Isotype (iso-PE) and mouse anti-goat-FITC controls are shown as grey histograms. Percent positive and relative fluorescent index (RFI) of MFI cytokine/MFI media is reported for each histogram. (C) Flow cytometric detection of IL13R alpha2 for D283 cells engineered to express IL13R alpha2 (D283-IL13R alpha2; blue histogram) and D283 parental (black histogram) stained with AF146 or B-D13-PE antibodies. All data are representative of more than three experiments each. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pone.0095123), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Human IL-13 R alpha 2 Antibody by Flow Cytometry
Cytokine induction of B-D13 antigen is dependent on mRNA transcription and translation.(A) IL13R alpha2 mRNA levels quantified by qPCR for U251T, PBT003-4, PBT008, and THP-1 cells after overnight culture in media, TNF/IL-4, or TNF/IL-13. mRNA levels were normalized to housekeeping genes (ACTB, UBC, GAPDH and RPLP0). (B, C) B-D13-PE immunoreactivity of PBT008 and THP-1 cells treated with increasing concentrations of (B) transcription blocker Actinomycin D (ActD) (0, 0.06, 0.1, 0.3 µg/ml) or (C) translation blocker Cycloheximide (CHX) (0, 2.5, 5, 10 µg/ml), then either cultured in media alone (black histograms) or stimulated with TNF/IL-4 (red histograms) for 5 hours. All data are representative of 2 separate experiments. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24787244), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of IL-13R alpha 2 by Flow Cytometry
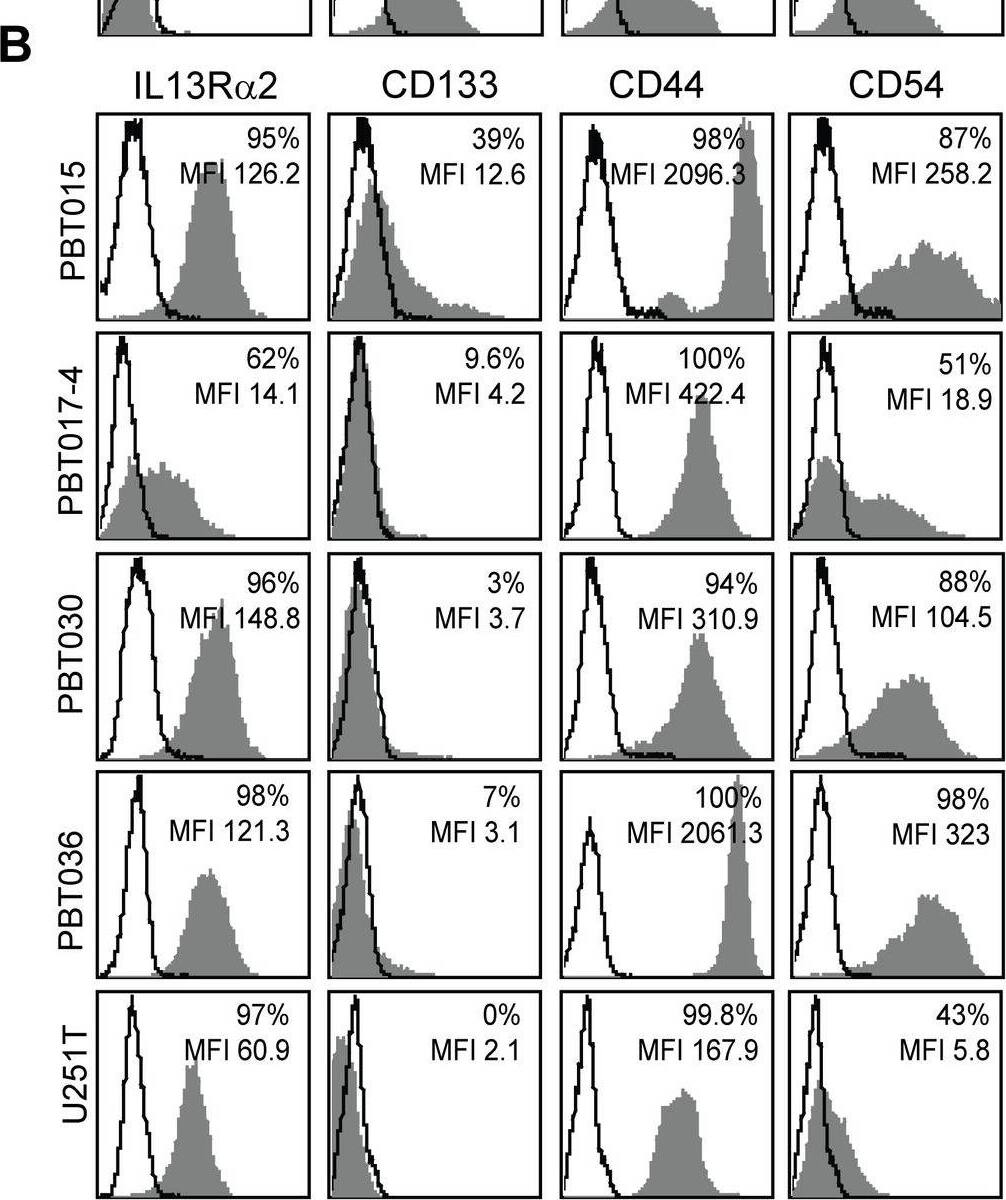
IL13R alpha2 expression is associated with mesenchymal gene expression in low-passage primary glioma cell lines.Flow cytometry analyses of (A) IL13R alpha2-negative glioma cell lines (PBT003-4, PBT008, PBT009, PBT022), and (B) IL13R alpha2-positive glioma cell lines (PBT015, PBT017-4, PBT030, PBT036, and U251T) for expression of the proneural marker CD133 and mesenchymal markers CD44 and CD54/ICAM-1 (grey histograms). Solid lines show isotype and secondary control antibodies. (C) Comparison of mean fluorescence intensity (MFI) of IL13R alpha2-negative and IL13R alpha2-positive glioma cell lines shown in panels A and B. (D-E) Distribution plots of Affymetrix gene array expression analyses of (D) IL13R alpha2-negative and (E) IL13R alpha2-positive glioma cell lines for mesenchymal, classical, neural and proneural signature gene expression. (F) Correlation of mesenchymal (MES) versus proneural (PN) average gene expression with RMA normalized IL13R alpha2 expression in glioma cell lines. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/24204956), licensed under a CC-BY license. Not internally tested by R&D Systems.