Human MMP-8 Antibody
R&D Systems, part of Bio-Techne | Catalog # MAB908

Key Product Details
Species Reactivity
Validated:
Cited:
Applications
Validated:
Cited:
Label
Antibody Source
Product Specifications
Immunogen
Phe21-Gly467
Accession # AAZ38714
Specificity
Clonality
Host
Isotype
Scientific Data Images for Human MMP-8 Antibody
Detection of Human MMP-8 by Western Blot
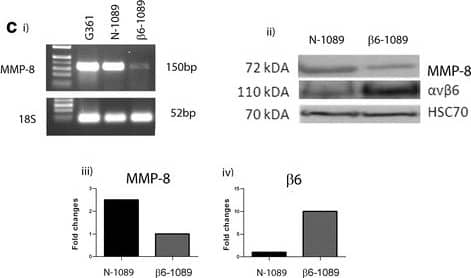
MMP-8 expression in; primary normal and DCIS myoepithelial cells and a cell line model. a Normal and ductal carcinoma in situ (DCIS) tissue immunohistochemically stained for matrix metalloproteinase-8 (MMP-8) (Atlas, HPA02122, 1:1000). Clear MMP-8 staining can be observed in the normal myoepithelial cells (MECs), which is absent in the DCIS section. Magnification × 400. b Normal MECs and luminal epithelial cells (LECs) were isolated from reduction mammoplasty tissue and DCIS MEC and DCIS LEC were isolated from a patient with high-grade DCIS. Tissues were enzymatically digested and magnetic bead separation was used to isolated specific populations (MEC - ITGB4, LEC - EpCAM). RNA was extracted from the isolated cells and G361 cells (a positive control for MMP-8), converted to cDNA by reverse transcription and then subjected to nested PCR for MMP-8 or PCR of housekeeping gene 18S. The MMP-8 PCR produces a product of 150 bp in normal MEC and G361 lanes, and other lanes only give a weak band. 18S gives a size of 52 bp in all sample lanes. The water (H2O) control lane shows no band. c (i) RNA was extracted from cell lines; G361, N-1089 and beta6-1089 and converted to cDNA as above. The cDNA was subjected to nested PCR for MMP-8 and bands were identified in G361 and N-1089 but only a weak band was observed in the beta6-1089 lane. All samples demonstrated a band for 18S. (ii) RIPA protein lysates were produced from N-1089 and beta6-1089 cells and 20 ug of protein was run on 8% PAGE then transferred to nitrocellulose and probed for MMP-8 (R&D Systems, mAb 908) alphav beta6 (Santa Cruz Biotechnology, SC-6632) or HSC70 as a loading control. The gel indicates that N-1089 expresses higher levels of MMP-8 and much lower levels of alphav beta6. While beta6-1089 exhibit much higher levels of alphav beta6 and lower levels of MMP-8. (iii) Densitometry of western blots indicates that MMP-8 expression in N-1089 is 2.5-fold higher than N-1089 and (iv) alphav beta6 expression in beta6-1089 is 10-fold higher than N-1089 Image collected and cropped by CiteAb from the following publication (https://breast-cancer-research.biomedcentral.com/articles/10.1186/s13058-017-0822-9), licensed under a CC-BY license. Not internally tested by R&D Systems.Applications for Human MMP-8 Antibody
Immunoprecipitation
Sample: Conditioned cell culture medium spiked with Recombinant Human MMP-8 (Catalog # 908-MP), see our available Western blot detection antibodies
Western Blot
Sample: Recombinant Human MMP-8 Western Blot Standard (Catalog # WBC017)
Human MMP-8 Sandwich Immunoassay
Reviewed Applications
Read 2 reviews rated 3.5 using MAB908 in the following applications:
Formulation, Preparation, and Storage
Purification
Reconstitution
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: MMP-8
Matrix metalloproteinases (MMPs) are a family of zinc and calcium dependent endopeptidases with the combined ability to degrade all the components of the extracellular matrix. MMP‑8 (neutrophil collagenase) is expressed in neutrophils, where it is stored in specific granules. MMP‑8 release from the neutrophils is stimulated by various factors such as interleukins 1 and 8, TNF-alpha and GM-CSF. MMP‑8 is capable of cleaving types I, II and III triple-helical collagen, gelatin peptides, fibronectin, proteoglycans, aggrecan, serpins, beta-casein and peptides such as angiotensin and substance P. In addition to its function in phagocytosis,
MMP‑8 has a high capacity for infiltrating connective tissue, and is implicated in the breakdown of the extracellular matrix in diseases such as rheumatoid arthritis. Structurally, MMP‑8 consists of several domains: a pro-domain that is cleaved upon activation, a catalytic domain containing the zinc-binding site, a short hinge region and a hemopexin-like domain. MMP‑8 is heavily glycosylated.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional MMP-8 Products
Product Documents for Human MMP-8 Antibody
Product Specific Notices for Human MMP-8 Antibody
For research use only