Human/Mouse Caspase-8 Antibody
R&D Systems, part of Bio-Techne | Catalog # AF1650

Key Product Details
Validated by
Species Reactivity
Validated:
Cited:
Applications
Validated:
Cited:
Label
Antibody Source
Product Specifications
Immunogen
Ser217-Asp384 (Asp285His) (p18 subunit), Leu385-Asp479 (p10 subunit)
Accession # Q14790
Specificity
Clonality
Host
Isotype
Scientific Data Images for Human/Mouse Caspase-8 Antibody
Detection of Human Caspase‑8 by Western Blot.
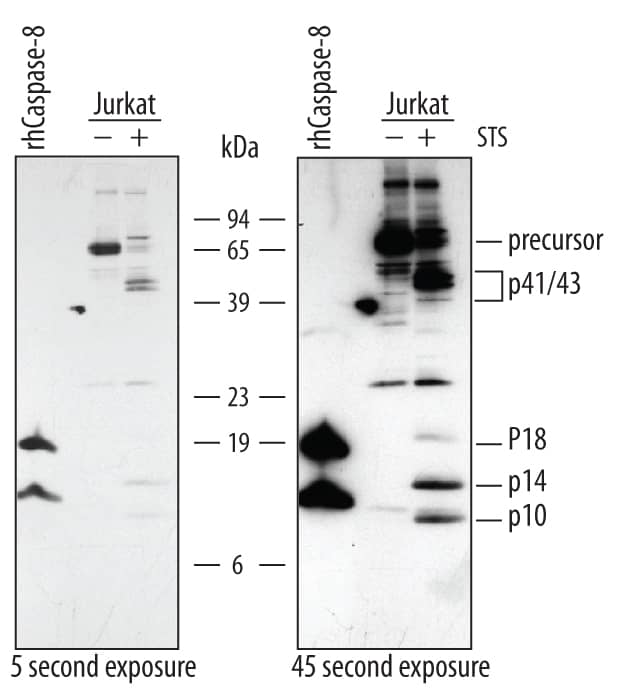
Western blot shows lysates of Jurkat human acute T cell leukemia cell line untreated (-) or treated (+) with 1 mM staurosporine (STS) for for 3 hours. PVDF membrane was probed with 0.5 µg/mL of Rabbit Anti-Human/Mouse Caspase-8 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1650), followed by HRP-conjugated Anti-Rabbit IgG Secondary Antibody (Catalog # HAF008). For additional reference Recombinant Human Caspase-8 (Catalog # 705-C8) was included. Specific bands were detected for Caspase-8 precursor at approximately 57-60 kDa (as indicated). In STS-treated samples, specific bands were detected for Caspase-8 p41/43 subunit at approximately 41 and 43 kDa (as indicated) and Caspase-8 p18, p14, and p10 subunits at approximately 18 kDa, 14 kDa, and 10 kDa, respectively (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 4.Detection of Human Caspase‑8 by Simple WesternTM.
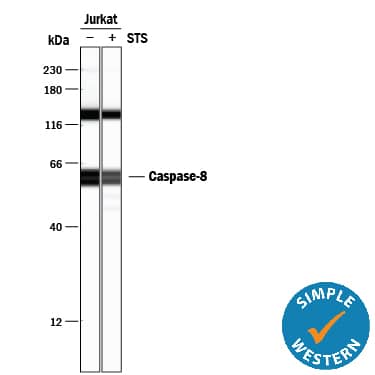
Simple Western lane view shows lysates of Jurkat human acute T cell leukemia cell line untreated (-) or treated (+) with 1 mM Staurosporine (STS) for 3 hours, loaded at 0.2 mg/mL. Specific bands were detected for Caspase‑8 at approximately 58-62 kDa (as indicated) using 5 µg/mL of Rabbit Anti-Human/Mouse Caspase‑8 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1650). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.Detection of Human Caspase-8 by Western Blot
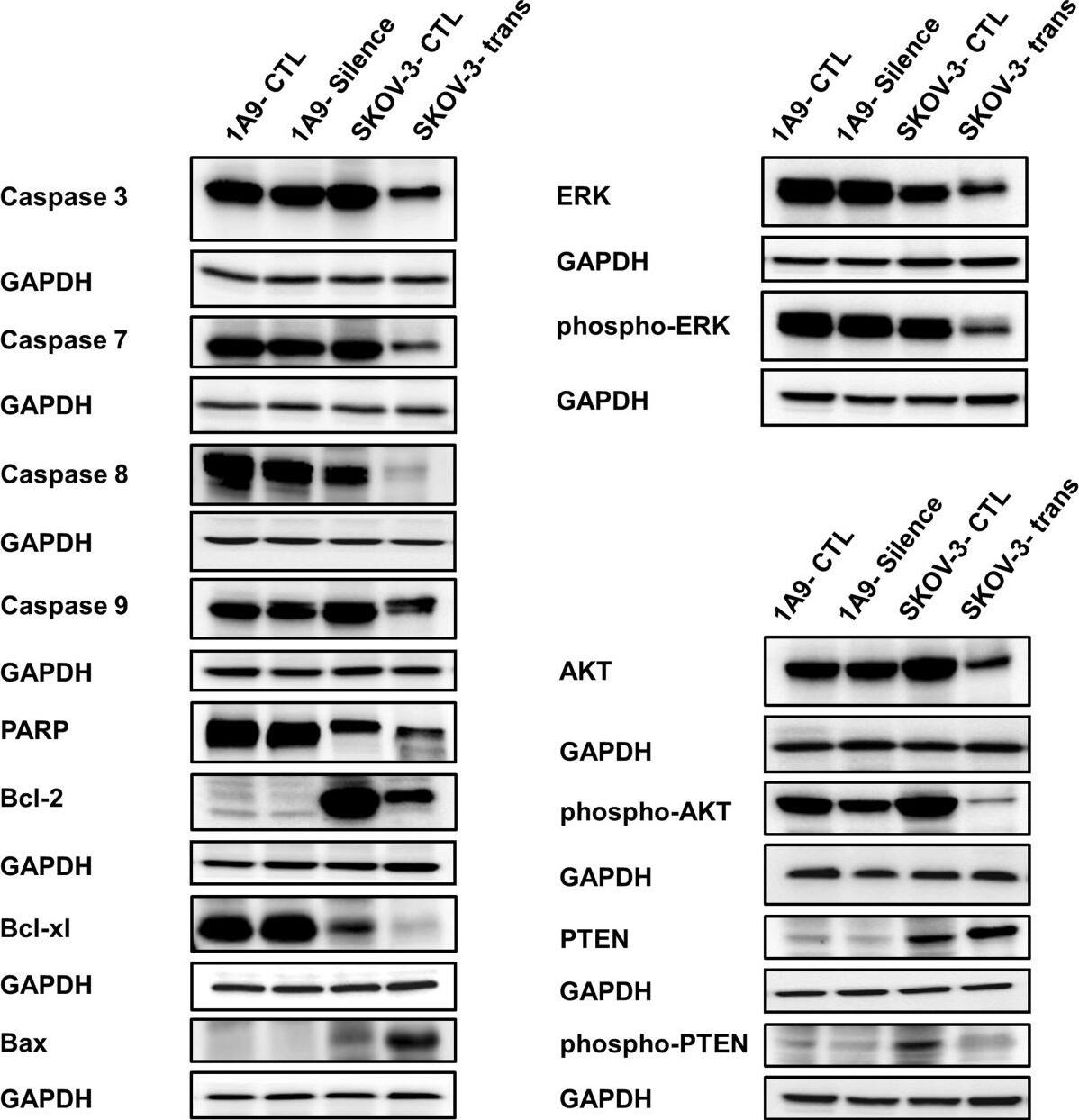
Western blot analysis of the expression of a number of proteins impacting proliferation and survival of ovarian cancer cells 48 hours after alteration of the Spry1 expression. Results indicate activation of apoptotic processes detected as overexpression of the pro-apoptotic Bax, decreased expression of the antiapoptotic proteins Bcl-2 and Bcl-xl, attenuation of procaspases 3, 7, 8 and 9 and cleavage of PARP in the Spry1-transfected SKOV-3 cells (left). Moreover, reduced expression of the activated forms of ERK and AKT along with increased expression of PTEN with concomitant decrease of phospho-PTEN (right) implicates repression of ERK as well as of AKT, with involvement of PTEN for the latter, in Spry1-induced inhibition of cell proliferation and survival. No significant change in the expression pattern of these proteins was found in the Spry1-silenced 1A9 cells. Image collected and cropped by CiteAb from the following publication (https://ovarianresearch.biomedcentral.com/articles/10.1186/1757-2215-7-61), licensed under a CC-BY license. Not internally tested by R&D Systems.Applications for Human/Mouse Caspase-8 Antibody
Simple Western
Sample: Jurkat human acute T cell leukemia cell line treated with Staurosporine (STS)
Western Blot
Sample: Jurkat human acute T cell leukemia cell line treated with staurosporine
Reviewed Applications
Read 1 review rated 4 using AF1650 in the following applications:
Formulation, Preparation, and Storage
Purification
Reconstitution
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Caspase-8
Caspase-8 (Cysteine-aspartic acid protease 8/Casp8a; also named MCH5, FLICA and MACH alpha1) is a 28 kDa member of the peptidase C14A family of enzymes (1, 2, 3). It is widely expressed and is considered an initiating caspase for the apoptotic cascade (4). Caspase-8 acts on a wide variety of substrates, including procaspases-3, 4, 6, 7, 9 and 10, c-FLIPL and procaspase-8 itself (1, 5, 6). Human procaspase-8a is a 54‑56 kDa, 479 amino acid (aa) protein (4, 7, 8, 9). It contains two N-terminal death domains (aa 1‑177), followed by a catalytic site that utilizes His317Gly318 plus Cys360. Normally, it is an inactive, cytosolic monomer (1, 10, 11). But following death-domain (DD) containing receptor oligomerization, Caspase-8 is recruited to the death-inducing signaling complex (DISC) that forms around the death domains of the oligomerized receptor (12). FADD/CAP-1 is recruited first, followed by procaspase-8/CAP-4 and, possibly, c-FLIPL and procaspase-10 (12). The recruitment, or concentration, of procaspase-8 induces homodimerization. This act alone is sufficient for activation. However, the activity level is modest at best, and appears to be directed towards either itself, or c-FLIPL, which is known to form a functional heterodimer with procaspase-8 (5, 11). When directed towards itself, autocleavage occurs first between Asp374Ser375, generating a 43 kDa (p43) N-terminal (aa 1‑374) and an 11 kDa C-terminal (aa 375 - 479) fragment. The C-terminus is further cleaved between Asp384Leu385 to generate a mature p10 subunit (aa 385‑479). The p43 subunit is next cleaved twice, once between Asp216Ser217, and again between Asp210Ser211 to generate a 26 kDa DD-containing prodomain (aa 1‑210) with an additional 18 kDa mature p18 subunit (aa 217‑374) (12). p18 and p10 noncovalently associate to form a 28 kDa heterodimer, which subsequently associates with another p18:p10 heterodimer to form an active, mature Caspase-8 molecule. This leaves the DISC to act on downstream apoptotic procaspases. In the event procaspase-8 comes to the DISC complexed with c-FLIPL, c-FLIPL will be cleaved by procaspase-8, generating a p43 fragment that is analogous to the Caspase-8 p43 subunit. This fragment, however, appears not to be an intermediate in a proteolytic cascade. Rather, it serves as a functional subunit, interacting with TRAF2 and activating NF kappaB. This may account for many of the nonapoptotic activities associated with Caspase-8 (5, 6, 13). Mature human and mouse Caspase-8a heterodimers are 73% aa identical (14).
References
-
Chowdhury, I. et al. (2008) Comp. Biochem. Physiol. B 151:10.
-
Boatright, K.M. & G.S. Salvesen (2003) Curr. Opin. Cell Biol. 15:725.
-
Launay, S. et al. (2005) Oncogene 24:5137.
-
Srinivasula, S.M. et al. (1996) Proc. Natl. Acad. Sci. USA 93:14486.
-
Hughes, M.A. et al. (2009) Mol. Cell 35:265.
-
Lamkanfi, M. et al. (2007) Cell Death Differ. 14:44.
-
Fernandes-Alnemri, T. et al. (1996) Proc. Natl. Acad. Sci. USA 93:7464.
-
Boldin, M.P. et al. (1996) Cell 85:803.
-
Muzio, M. et al. (1996) Cell 85:817.
-
Donepudi, M. et al. (2003) Mol. Cell 11:543.
-
Boatright, K.M. et al. (2003) Mol. Cell 11:529.
-
Golks, A. et al. (2006) Cell Death Differ. 13:489.
-
Scaffidi, C. et al. (1997) J. Biol. Chem. 272:26953.
-
Sakamaki, K. et al. (1998) Eur. J. Biochem. 253:399.
Alternate Names
Gene Symbol
UniProt
Additional Caspase-8 Products
Product Documents for Human/Mouse Caspase-8 Antibody
Product Specific Notices for Human/Mouse Caspase-8 Antibody
For research use only