Human PRELP Antibody
R&D Systems, part of Bio-Techne | Catalog # MAB6447

Key Product Details
Species Reactivity
Applications
Label
Antibody Source
Product Specifications
Immunogen
Gln21-Ile382
Accession # P51888
Specificity
Clonality
Host
Isotype
Scientific Data Images for Human PRELP Antibody
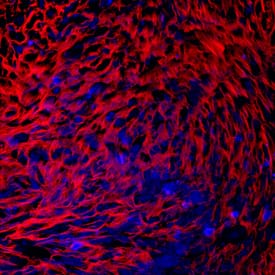
PRELP in Human Chondrocytes.
PRELP was detected in immersion fixed human mesenchymal stem cells differentiated into chondrocytes using Mouse Anti-Human PRELP Monoclonal Antibody (Catalog # MAB6447) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Mouse IgG Secondary Antibody (red; Catalog # NL007) and counterstained with DAPI (blue). Specific staining was localized to cytoplasm. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.Applications for Human PRELP Antibody
Immunocytochemistry
Sample: Immersion fixed human mesenchymal stem cells differentiated into chondrocytes
Reviewed Applications
Read 1 review rated 4 using MAB6447 in the following applications:
Formulation, Preparation, and Storage
Purification
Reconstitution
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: PRELP
PRELP (Proline aRginine-rich End Leucine-rich repeat Protein; also Prolargin) is a 55‑62 kDa secreted glycoprotein that belongs to the small leucine-rich proteoglycan (SLRP) superfamily of extracellular matrix (ECM) molecules (1‑4). Within this family, it is considered a class II member, implying that it is unlikely to form dimeric structures (3). PRELP is synthesized as a 382 amino acid (aa) precursor that contains a 20 aa signal sequence plus a 362 aa mature region (1, 5). Like other SLRPs, PRELP contains an N-terminal extension (aa 72‑107) coupled to multiple Leu-rich repeats (LRRs) (aa 95‑382) (6). Unlike other SLRPs, PRELP does not contain any proteoglycan chains, and its N‑terminal extension is highly basic in charge. The N-terminus reportedly binds to negatively-charged heparin/heparin-sulfate, chondroitin sulfate, and Gram- bacterial cell walls, while the LRR region participates in protein-protein interactions (7‑9). Although PRELP is known to be synthesized by only a few cell types, including osteoblasts, skeletal muscle and chondrocytes, its expression is likely to be more widespread, given its presence in the basement membrane (BM) of Bowman’s capsule, epididymal epithelium and the stratified squamous epithelium of the skin (1, 10, 11). The dual binding profile of PRELP is key to its function. In cartilage, PRELP likely links chondrocyte cell membrane heparin sulfate (HS) chains to endogenous type II collagen. Within the context of the BM, PRELP likely plays an anchoring role. The BM is composed of type IV collagen and laminin, linked together by nidogen. BM Perlecan reinforces this linkage by binding to all three components. PRELP, on the edge of the BM, can bind to free perlecan HS chains (via its N-terminus), and to underlying type I collagen (via its LRRs), thus forming an anchor for the BM (11). Notably, the N-terminus appears to do more than simply provide part of a linkage mechanism. In bone, osteoblast secreted PRELP is hypothesized to undergo proteolysis by enzymes such as LysC and glutamyl endopeptidase. This will generate 40‑75 aa N‑terminal fragments that can bind to chondroitin sulfate adducts that exist on the surface of prefusion osteoclast precursors. Following binding, PRELP is internalized, complexed to annexin-II, and translocated to the nucleus, where it interacts with NF kappaBp65 to block osteoclast maturation (8). In tissue, PRELP may also undergo proteolytic processing during inflammation to release an N‑terminal fragment containing aa 21‑42 of the precursor (7). This sequence has been shown to possess potent antimicrobial activity by creating pores in bacterial cell walls. Mature human PRELP shares 91% aa identity with mouse PRELP (10).
References
- Bengtsson, E. et al. (1995) J. Biol. Chem. 270:25639.
- Merline, R. et al. (2009) J. Cell Commun. Signal. 3:323.
- McEwan, P.A. et al. (2006) J. Struct. Biol. 155:294.
- Neame, P.J. et al. (1999) Cell. Mol. Life Sci. 55:1327.
- Grover, J. et al. (1996) Genomics 38:109.
- SwissProt # P51888.
- Bengtsson, E. et al. (2000) J. Biol. Chem. 275:40695.
- Rucci, N. et. al. (2009) J. Cell Biol. 187:669.
- Malmsten, M. et al. (2006) Matrix Biol. 25:294.
- Grover, J. & P.J. Roughley (2001) Matrix Biol. 20:555.
- Bengtsson, E. et al. (2002) J. Biol. Chem. 277:15061.
Long Name
Alternate Names
Entrez Gene IDs
Gene Symbol
UniProt
Additional PRELP Products
Product Documents for Human PRELP Antibody
Product Specific Notices for Human PRELP Antibody
For research use only