Mouse LBP Antibody
R&D Systems, part of Bio-Techne | Catalog # AF6635

Key Product Details
Species Reactivity
Applications
Label
Antibody Source
Product Specifications
Immunogen
Gly25-Val481 (Ser102Arg, Tyr284His)
Accession # NP_032515
Specificity
Clonality
Host
Isotype
Scientific Data Images for Mouse LBP Antibody
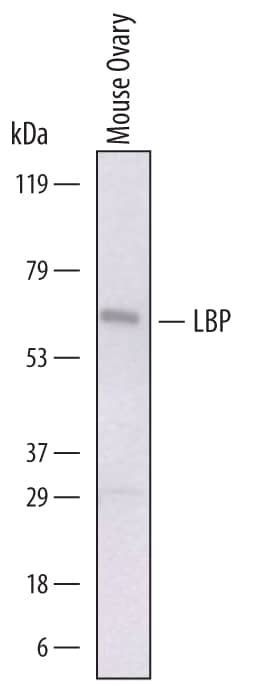
Detection of Mouse LBP by Western Blot.
Western blot shows lysates of mouse ovary tissue. PVDF membrane was probed with 1 µg/mL of Sheep Anti-Mouse LBP Antigen Affinity-purified Polyclonal Antibody (Catalog # AF6635) followed by HRP-conjugated Anti-Sheep IgG Secondary Antibody (Catalog # HAF016). A specific band was detected for LBP at approximately 65 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.Applications for Mouse LBP Antibody
Western Blot
Sample: Mouse ovary tissue
Formulation, Preparation, and Storage
Purification
Reconstitution
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: LBP
LBP (Lipopolysaccharide binding protein) is a 58‑62 kDa, single-chain glycoprotein member of the BPI/LBP family, BPI/PLUNC/PSP superfamily of lipid-binding proteins (1-3). It is secreted by a number of mammalian cell types, including hepatocytes (4), gingival keratinocytes (5), intestinal Paneth cells (6), and type II Greater alveolar cells (7). LBP is considered to be a class 1 APR (acute phase reactant) that is induced upon exposure to both IL-1 and IL-6 (8). These two cytokines appear upon immune cell exposure to pathogenic microbes. Following its synthesis and release, LBP is known to interact with bacterial wall components, lipopolysaccharide/LPS/Lipid A from Gram- (Gm-) bacteria, and lipoteichoic acid/LTA from Gm+ bacteria (9-13). In the case of LPS, this interaction appears to occur both in the bacterial cell wall, and within the intercellular space, where LPS micelles naturally form following bacterial death and cell wall dissolution (14-17). LBP is posited to induce disassembly of LPS micelles, allowing for LPS binding to LBP, and a heparin-mediated transfer of LPS from LBP to membrane-bound CD14 on the surface of monocytes/macrophages (15, 18). This CD14:LPS complex activates a TLR4:MD2 membrane complex, resulting in the production of NO and TNF-alpha (19). TNF-alpha serves as a chemoattractant for PMNs, and an initiator of coagulation that helps to wall-off and localize microbial elements (16). Notably, increased concentrations of LBP are also associated with parasitic infections (Trypanosoma), and may contribute to the immune response towards parasites (20). In addition to the above, LBP is also reported to transfer LPS to lipoproteins, particularly HDL and LDL (19, 21-23). For LDL, this transfer appears to be inhibitory to monocyte activation; for HDL, the effect may be either stimulatory or inhibitory, depending upon the circumstances (19). Mouse LBP is synthesized as a 481 amino acids (aa) precursor that contains a 25 aa signal sequence and a 456 aa mature region (aa 26-481) (24). It contains an N‑terminal LPS binding region plus a likely C-terminal LPS transfer region (24, 25). Mature mouse LBP shares 68% and 88% aa identity with human and rat LBP, respectively (11, 25).
References
- Beamer, L.J. et al. (1998) Protein Sci. 7:906.
- Schroder, N.W.J. & R.R. Schumann (2005) J. Endotoxin Res. 11:237.
- Miyake, K. (2006) J. Endotoxin Res. 12:195.
- Grube, B.J. et al. (1994) J. Biol. Chem. 269:8477.
- Ren, L. et al. (2004) J. Periodont. Res. 39:242.
- Hansen, G.H. et al. (2009) Histochem. Cell Biol. 131:727.
- Dentener, M.A. et al. (2000) Am. J. Respir. Cell Mol. Biol. 23:146.
- Schumann, R.R. et al. (1996) Mol. Cell. Biol. 16:3490.
- Weber, J.R. et. al. (2003) Immunity 19:269.
- Schroder, N.W.J. et al. (2004) J. Immunol. 173:2683.
- Su, G.L. et al. (1994) J. Immunol. 153:743.
- Schroder, N.W.J. et al. (2003) J. Biol. Chem. 178:15587.
- Wright, S.D. et al. (1989) J. Exp. Med. 170:1231.
- Hallatschek, W. et al. (2004) Eur. J. Immunol. 34:1441.
- Schumann, R.R. & E. Latz (2000) Chem. Immunol. 74:42.
- Mannel, D.N. & B. Echtenacher (2000) Chem. Immunol. 74:141.
- Tsukamoto, H. et al. (2010) Int. Immunol. 22:271.
- Heinzelmann, M. & H. Bosshart (2005) J. Immunol. 174:2280.
- Gallay, P. et al. (1993) Infect. Immun. 61:378.
- Ngure, R.M. et al. (2009) Res. Vet. Sci. 86:394.
- Levels, J.H.M. et al. (2005) Infect. Immun. 73:2321.
- Hubacek, J.A. et al. (1997) Biochem. Biophys. Res. Commun. 236:427.
- Thompson, P.A. & R.L. Kitchens (2006) J. Immunol. 177:4880.
- Lengacher, S. et al. (1995-1996) J. Inflamm. 47:165.
- Schumann, R.R. et al. (1990) Science 249:1429.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional LBP Products
Product Documents for Mouse LBP Antibody
Product Specific Notices for Mouse LBP Antibody
For research use only