Kit Summary
Complete, fetal bovine serum-containing media to support the expansion of mesenchymal stem cells (MSCs) under GMP-grade culture conditions.
Key Benefits
- Produced according to GMP Guidelines
- All components, including fetal bovine serum, were selected and optimized for MSCs
- Formulated to reduce variation
- Ready to use
- Additional testing and documentation is available upon request
Why Culture MSCs GMP-grade Medium?
Mesenchymal stem cells (MSC) hold significant promise for tissue engineering and regenerative medicine. The use of MSCs for translational research requires their expansion in GMP-grade media and, depending on the application, GMP-grade cytokines and growth factors. GMP-grade media and proteins are manufactured so that all products, materials, equipment, and processes used in production are traceable and controlled. This extra level of traceability is important and necessary for transitioning MSC research into a translational, FDA-approved therapy.
Our StemXVivo® GMP Mesenchymal Stem Cell Expansion Media is the same formulation as our StemXVivo® Mesenchymal Expansion Media (Catalog # CCM004), but is produced according to relevant sections of the following documents: WHO TRS, No. 822, 1992 Annex 1, Good Manufacturing Practices for Biological Products; USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue Engineered Products and USP Chapter 92, Growth Factors and Cytokines Used in Cell Therapy Manufacturing.
StemXVivo® GMP Mesenchymal Stem Cell Expansion Media:
Kit Contents
StemXVivo® GMP Mesenchymal Stem Cell Expansion Media is supplied in a 250 mL volume and contains high quality factors to support MSC expansion in vitro. Contains fetal bovine serum.
Stability and Storage
Store unopened reagents in the dark at -20 °C in a manual defrost freezer. Do not use past the expiration date.
Precaution
When handling biohazardous materials such as human cells, safe laboratory procedures should be followed and protective clothing should be worn.
Limitations
- FOR LABORATORY RESEARCH USE ONLY. NOT FOR USE IN DIAGNOSTIC PROCEDURES.
- The safety and efficacy of this product in diagnostic or other clinical uses has not been established.
- This reagent should not be used beyond the expiration date indicated on the label.
- Results may vary due to variations among cells derived from different donors.
Data Examples
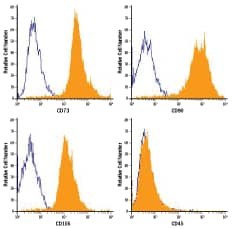
| Figure 1: Phenotypic Analysis of Human MSCs Expanded in MSC Expansion Media. Human MSCs were expanded using StemXVivo® GMP MSC Expansion Media. Filled histograms indicate cells stained with markers of undifferentiated MSCs including anti-CD105 (R&D Systems, Catalog # FAB1097A), anti-CD73 (R&D Systems, Catalog # FAB5795P), anti-CD90 (R&D Systems, Catalog # FAB2067A), or anti-CD45 (R&D Systems, Catalog # FAB1430P). The open histograms show isotype-matched control staining. MSCs appropriately express high levels of CD105, CD73, and CD90, and lack expression of CD45. |
GMP MEDIA
R&D Systems' GMP media is produced according to relevant sections of the following documents: WHO TRS, No. 822, 1992 Annex 1, Good Manufacturing Practices for Biological Products; USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue Engineered Products and USP Chapter 92, Growth Factors and Cytokines Used in Cell Therapy Manufacturing.
R&D Systems' quality focus includes:
- Manufacturing and testing under an ISO 9001:2008 and ISO 13485:2003 certified quality system
- Documented processes and QA control of documentation and process changes
- Personnel training programs • Raw material testing and vendor qualification/monitoring
- Fully validated equipment, processes and test methods
- Equipment calibration schedules using a computerized calibration program
- Facility maintenance, safety programs and pest control
- Material review process for variances
- Monitoring of stability over product shelf life
Additional testing and documentation requested by the customer can be arranged at an additional cost.
Production records and facilities are available for examination by appropriate personnel on site at R&D Systems in Minneapolis, Minnesota USA. R&D Systems sells its GMP grade media products for research use or further manufacturing use in ex vivo cell therapy applications. They are not for in vivo use or for use as therapeutic or other drugs, biologic products or devices. Please read the following End User Terms prior to using this product.
END USER TERMS OF USE OF PRODUCT
The following terms are offered to you upon your acceptance of these End User Terms of Use of Product. By using this product, you indicate your acknowledgment and agreement to these End User Terms of Use of Product. If you do not agree to be bound by and comply with all of the provisions of these End User Terms of Use of Product, you should contact your supplier of the product and make arrangements to return the product.
We suggest you print and retain a copy of these End User Terms of Use of Product for your records.
The End User is aware that R&D Systems, Inc. sells its GMP products for research use only or further manufacturing and not for in vivo use, the production of therapeutics or other drugs or for biologic products or devices. The End User further agrees, as a condition of the sale of R&D Systems' GMP products that: a) the End User will not use this GMP Product in any procedure wherein the product may be directly or indirectly administered to humans, unless the End User has obtained, or prior to their use will have obtained, an Investigational New Drug (IND) exemption from the FDA and will use the product only in accordance with the protocols of such IND and of the Institutional Review Board overseeing the proposed research, or b) the End User will use the products outside of the United States in accordance with the protocols of research approved by the Institutional Review Board or authorized ethics committee and regulatory agencies to which the End User is subject to in their territory.
R&D Systems, Inc. has the right, at its sole discretion, to modify, add or remove any terms or conditions of these End User Terms of Use without notice or liability to you. Any changes to these End User Terms of Use are effective immediately following the printing of such changes on this product insert. The most recent version of these End User Terms of Use of Product may be found at: RnDSystems.com/Legal.
You agree to review these End User Terms of Use of Product to ensure any subsequent use by you of R&D Systems' GMP Products following changes to these End User Terms of Use of Product constitutes your acceptance of all such changes.
The term 'mesenchymal stem cells' (MSCs) is most commonly used to describe multipotent self-renewing cells that can be differentiated in vitro to generate adipocytes, chondrocytes, and osteoblasts. However, because these biological properties and hierarchical relationships remain to be clearly demonstrated in vivo, the term 'multipotent mesenchymal stromal cells' is often used to distinguish cultured cells from their in vivo precursors. Originally discovered in mouse bone marrow, multipotent mesenchymal stromal cells cultured from a variety of species and tissue types, have been shown to differentiate into progeny of additional lineages including, cardiomyocytes, endothelial cells, hepatocytes, and neural cells. Again, the physiological relevance of these findings remains to be determined.













