Recombinant Human EGFR Fc Chimera Protein, CF Best Seller
R&D Systems, part of Bio-Techne | Catalog # 344-ER

Key Product Details
Source
Accession #
Structure / Form
Conjugate
Applications
Product Specifications
Source
| Human EGFR (Leu25-Ser645) Accession # CAA25240.1 |
IEGRMD | Human IgG1-Fc (Pro100-Lys330) |
| N-terminus | C-terminus |
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
SDS-PAGE
Activity
Reviewed Applications
Read 2 reviews rated 4.5 using 344-ER in the following applications:
Scientific Data Images for Recombinant Human EGFR Fc Chimera Protein, CF
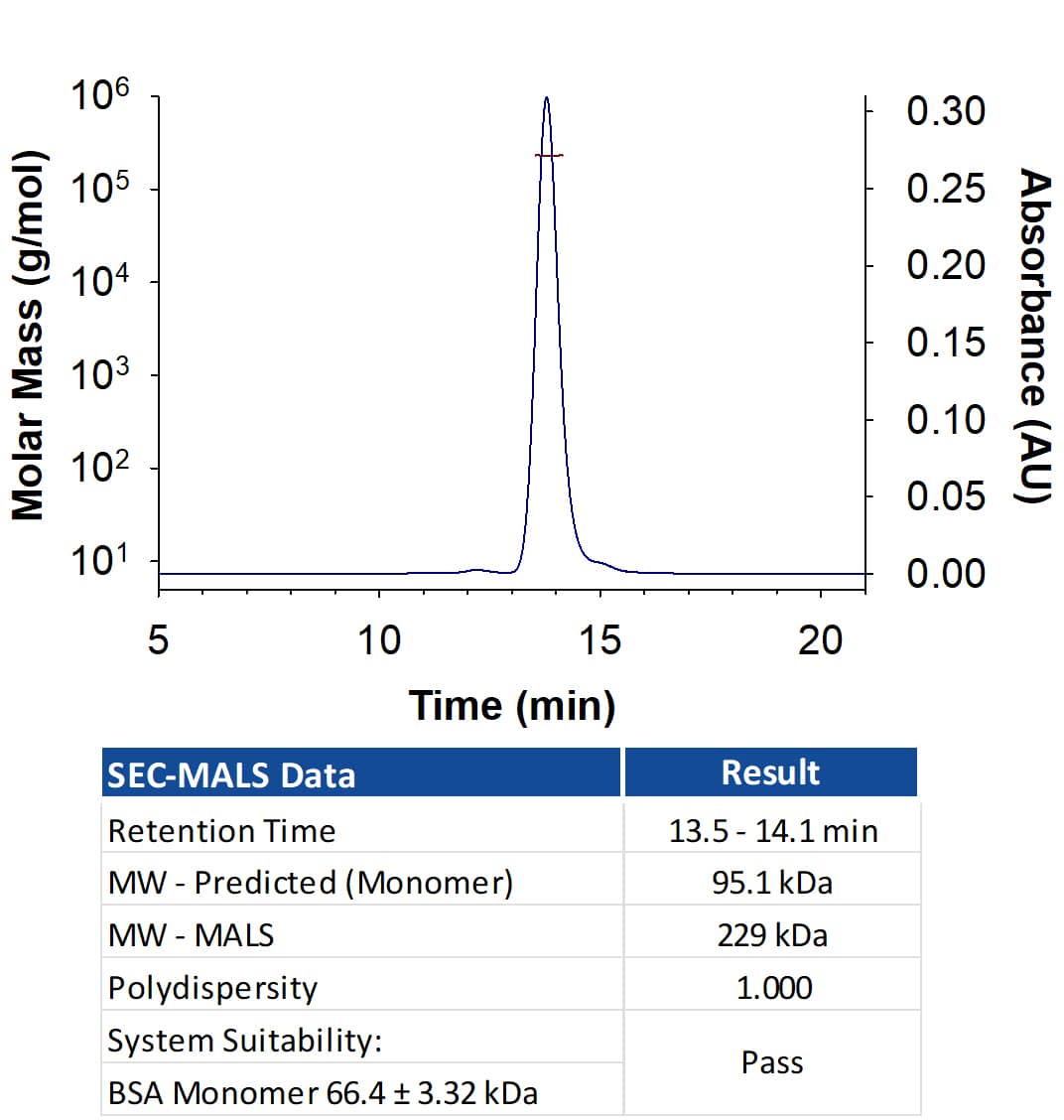
Recombinant Human EGFR Fc Chimera Protein SEC-MALS
Recombinant Human EGFR Fc Chimera (Catalog # 344-ER) has a molecular weight (MW) of 229 kDa as analyzed by SEC-MALS, suggesting that this protein is a homodimer. MW may differ from predicted MW due to post-translational modifications (PTMs) present (i.e. Glycosylation).Formulation, Preparation and Storage
344-ER
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS. |
| Reconstitution |
Reconstitute at 100 μg/mL in sterile PBS.
|
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: EGFR
The EGFR subfamily of receptor tyrosine kinases comprises four members: EGFR (also known as HER-1, ErbB1, or ErbB), ErbB2 (Neu, HER-2), ErbB3 (HER-3), and ErbB4 (HER-4). All family members are type I transmembrane glycoproteins with an extracellular ligand binding domain containing two cysteine-rich domains separated by a spacer region and a cytoplasmic domain containing a membrane-proximal tyrosine kinase domain followed by multiple tyrosine autophosphorylation sites (1, 2). The human EGFR cDNA encodes a 1210 amino acid (aa) precursor with a 24 aa signal peptide, a 621 aa extracellular domain (ECD), a 23 aa transmembrane segment, and a 542 aa cytoplasmic domain (3, 4). Soluble receptors consisting of the extracellular ligand binding domain are generated by alternate splicing in human and mouse (5‑7). Within the ECD, human EGFR shares 88% aa sequence identity with mouse and rat EGFR. It shares 43%-44% aa sequence identity with the ECD of human ErbB2, ErbB3, and ErbB4. EGFR binds a subset of the EGF family ligands, including EGF, amphiregulin, TGF-alpha, betacellulin, epiregulin, HB-EGF, and epigen (1, 2). Ligand binding induces EGFR homodimerization as well as heterodimerization with ErbB2, resulting in kinase activation, heterodimerization tyrosine phosphorylation and cell signaling (8‑12). EGFR can also be recruited to form heterodimers with the ligand‑activated ErbB3 or ErbB4. EGFR signaling regulates multiple biological functions including cell proliferation, differentiation, motility, and apoptosis (13, 14). EGFR is overexpressed in a wide variety of tumors and is the target of several anti-cancer drugs (15).
References
- Singh, A.B. and R.C. Harris (2005) Cell. Signal. 17:1183.
- Shilo, B.Z. (2005) Development 132:4017.
- Lin, C. et al. (1984) Science 224:843.
- Ullrich, A. et al. (1984) Nature 309:418.
- Reiter, J.L. and N.J. Maihle (1996) Nucleic Acids Res. 24:4050.
- Reiter J.L. et al. (2001) Genomics 71:1.
- Xu, Y.H. et al. (1984) Nature 309:806.
- Graus-Porta, D. et al. (1997) EMBO J. 16:1647.
- Yarden, Y. et al. (1987) Biochemistry 26:1434.
- Burgess, A.W. et al. (2003) Mol. Cell 12:541.
- Lemmon, M.A. et al. (1997) EMBO J. 16:281.
- Cohen, S. et al. (1982) J. Biol. Chem. 257:1523.
- Sibilia, M. and E.F. Wagner (1995) Science 269:234.
- Miettinen, P.J. et al. (1995) Nature 376:337.
- Roskoski Jr., R. (2004) Biochem. Biophys. Res. Commun. 319:1.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional EGFR Products
Product Documents for Recombinant Human EGFR Fc Chimera Protein, CF
Product Specific Notices for Recombinant Human EGFR Fc Chimera Protein, CF
For research use only