Recombinant Human IL-11 Protein Best Seller
R&D Systems, part of Bio-Techne | Catalog # 218-IL

Bigger Scale, More Savings. Try our NEW version of Human Recombinant IL-11 (10836-IL). Combining R&D Systems quality with scalability that allows for lower price points and a solid supply chain.
Key Product Details
Product Specifications
Source
Pro22-Leu199
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
SDS-PAGE
Activity
The ED50 for this effect is 0.02-0.12 ng/mL.
Scientific Data Images for Recombinant Human IL-11 Protein
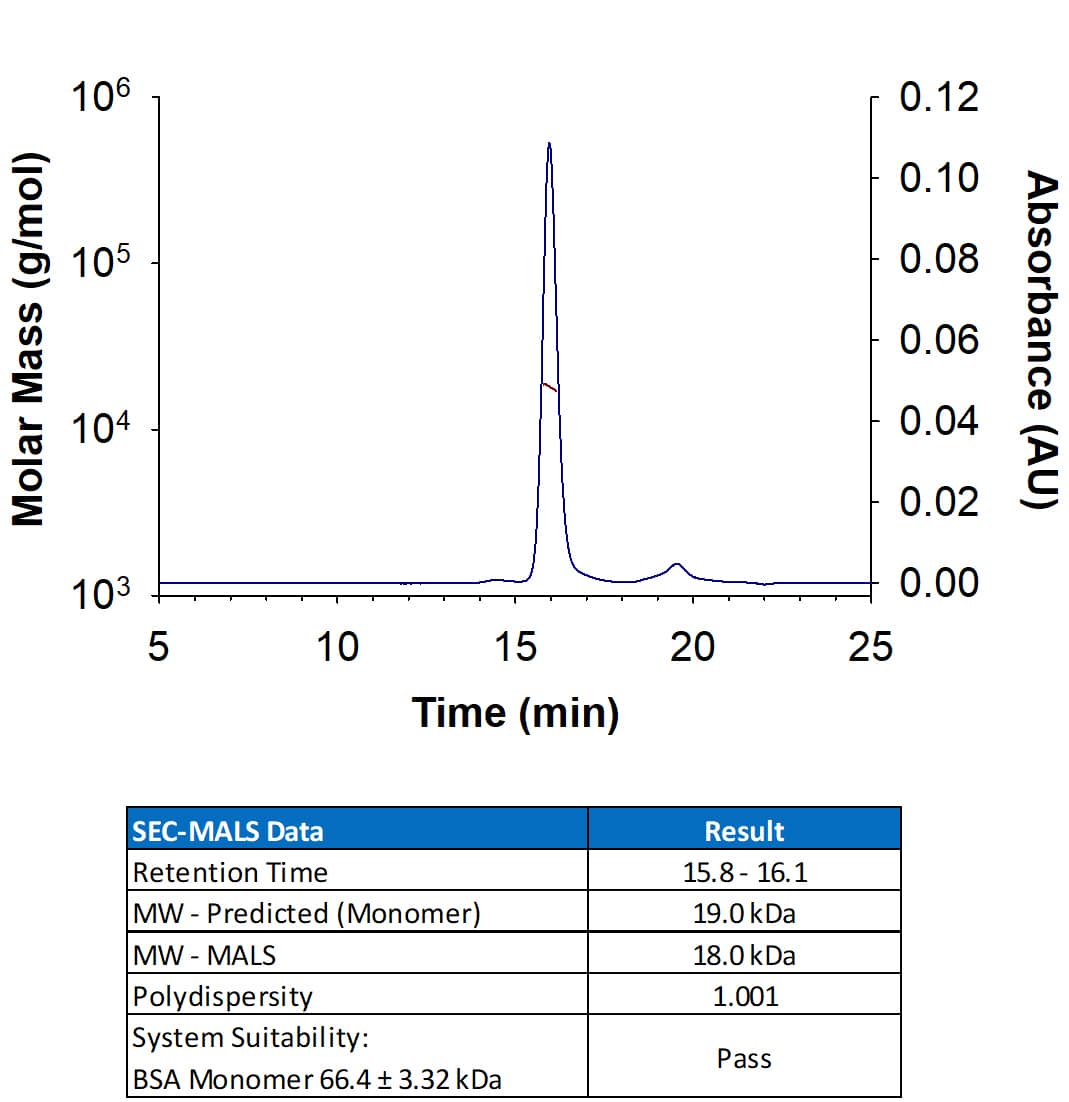
Recombinant Human IL‑11 Protein SEC-MALS.
Recombinant Human IL-11 Protein (Catalog # 218-IL) has a molecular weight (MW) of 18.0 kDa as analyzed by SEC-MALS, suggesting that this protein is a monomer. MW may differ from predicted MW due to post-translational modifications (PTMs) present (i.e. Glycosylation).Formulation, Preparation and Storage
Carrier Free
What does CF mean?CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
What formulation is right for me?In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
Carrier: 218-IL
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS and EDTA with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 50 μg/mL in sterile PBS containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Carrier Free: 218-IL/CF
| Formulation | Supplied as a 0.2 μm filtered solution in PBS and EDTA. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: IL-11
IL-11 (Interleukin 11) is a pleiotropic cytokine in the IL-6 family, which also includes LIF, CNTF, Oncostatin M, Cardiotrophin-1, IL-27 and IL-31 (1-3). In humans, IL-11 was also independently discovered as an adipogenesis inhibitory factor (AGIF) (3). The human IL-11 cDNA encodes a 199 amino acid (aa) precursor, which generates a 178 aa, 19 kDa mature unglycosylated protein. Mature human IL-11 shares 88%, 88%, and 96% aa sequence identity with mouse, rat and canine IL-11, respectively. IL-11 is secreted by osteoblasts, synoviocytes, fibroblasts, chondrocytes, intestinal myofibroblasts, and trophoblasts, among other cell types (1). It is found in the plasma mainly during inflammation, such as that associated with viral infection, cancer, or inflammatory arthritis, and is considered to be primarily anti‑inflammatory (1). It stimulates hematopoiesis and thrombopoiesis, regulates macrophage differentiation, and confers mucosal protection in the intestine (1). It has also been found to enhance T cell polarization toward Th2, promote B cell IgG production, increase osteoclast bone absorption, protect endothelial cells from oxidative stress, and regulate epithelial proliferation and apoptosis (1). IL-11 synergizes with several other cytokines to produce these effects, and its effects overlap with those of IL-6 (1). IL-11 receptor activation requires formation of a complex of two IL-11 molecules with two molecules of the ligand-binding IL-11 R alpha subunit and two molecules of the ubiquitously expressed cell signaling beta subunit, gp130 (4). A soluble form of IL-11 R alpha can bind IL-11 and either form a signaling complex with gp130 on the cell surface, or inhibit cell surface IL-11 R alpha/gp130 signaling (5-7).
References
- Putoczki, T. and M. Ernst (2010) J. Leukoc. Biol. 88:1109.
- Paul, S.R. et al. (1990) Proc. Natl. Acad. Sci. USA 87:7512.
- Kawashima, I. et al. (1991) FEBS Lett. 283:199.
- Barton, V.A. et al. (2000) J. Biol. Chem. 275:36197.
- Curtis, D.J. et al. (1997) Blood 90:4403.
- Baumann, H. et al. (1996) J. Immunol. 157:284.
- Karow, J. et al. (1996) Biochem. J. 318:489.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional IL-11 Products
Product Documents for Recombinant Human IL-11 Protein
Product Specific Notices for Recombinant Human IL-11 Protein
For research use only