Recombinant Human IL-35 Fc Chimera Protein, CF
R&D Systems, part of Bio-Techne | Catalog # 8608-IL

Key Product Details
Source
Conjugate
Applications
Product Specifications
Source
| Human IL-27 beta/EBI3 (Arg21-Lys229) Accession # Q14213 |
(GGGS)4 | Human IL-12 alpha/p35 Arg23-Ser219 Accession # P29459 |
IEGRMD | Human IgG1 Pro100-Lys330) |
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
SDS-PAGE
Activity
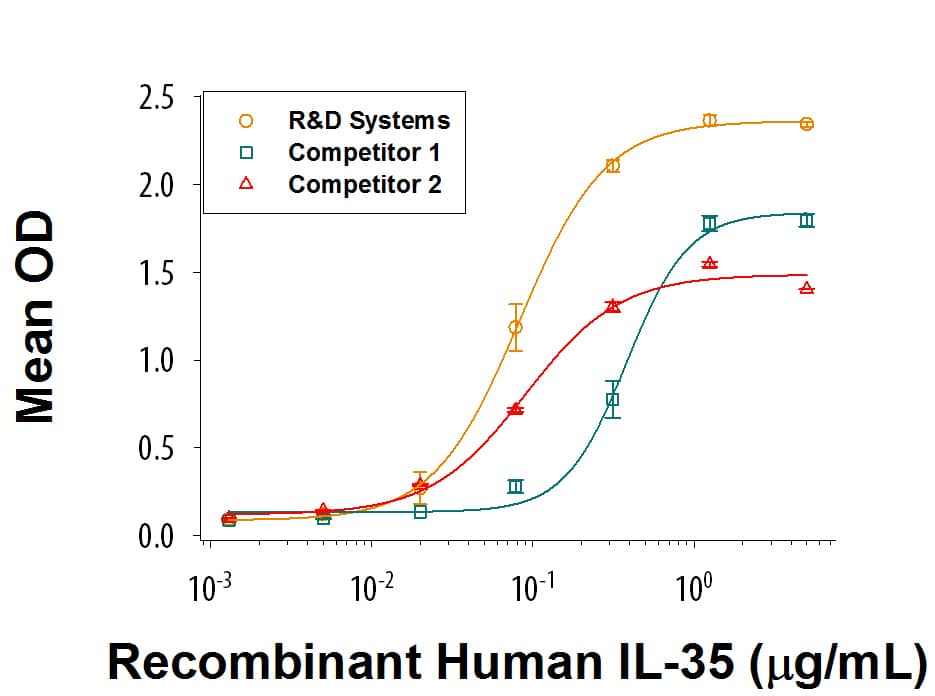
When Recombinant Human IL-12 R beta2 Fc Chimera (Catalog # 1959-B2) is immobilized at 5 μg/mL (100 μL/well), the concentration of Recombinant Human IL-35 Fc Chimera that produces 50% optimal binding response is approximately 20-120 ng/mL.
Scientific Data Images for Recombinant Human IL-35 Fc Chimera Protein, CF
Recombinant Human IL-35 Fc Chimera Protein Bioactivity
When Recombinant Human IL-12 R beta 2 Fc Chimera (Catalog # 1959-B2) is immobilized at 5 μg/mL (100 μL/well), Recombinant Human IL-35 Fc Chimera (Catalog # 8608-IL) binds with an ED50 of 20-120 ng/mL. Recombinant Human IL-35 from the two competitors have much weaker Recombinant Human IL-12 R beta 2 Fc Chimera binding activity.Recombinant Human IL-35 Fc Chimera Protein SDS-PAGE
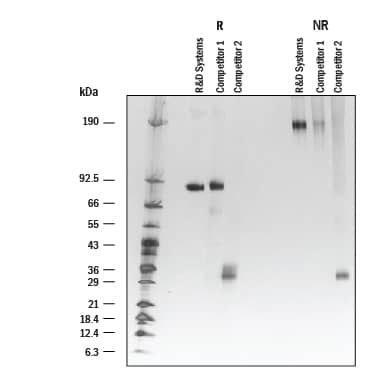
1 μg/lane of Recombinant Human IL-35 Fc Chimera Protein from R&D Systems and two competitors was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by silver staining. Recombinant Human IL-35 Fc Chimera Protein (Catalog # 8608-IL) from R&D Systems shows single bands at 85 kDa and 185 kDa, respectively. The R&D Systems Protein offers a better purity than the competition.Formulation, Preparation and Storage
8608-IL
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS. |
| Reconstitution |
Reconstitute at 100 μg/mL in PBS.
|
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: IL-35
Interleukin 35 (IL-35) is a member of the IL-12 family of heterodimeric cytokines. Unlike other IL-12 family cytokines which stimulate the immune response, the predominant function of IL-35 is as an immunosuppressant. IL-12 cytokines are composed of an alpha and beta subunit which, for IL-35 are the IL-12 p35 subunit and the EBI3 subunit, respectively (1-3). The IL-12 p35 subunit of IL-35 is synthesized as a 219 amino acid (aa) precursor protein with a 22 aa signal sequence and a 197 aa mature region. The EBI3 subunit of IL-35 is synthesized as a 229 aa precursor protein that contains a 20 aa signal sequence and a 209 aa mature region. Human and mouse IL-35 share 58% and 62% sequence homology in their IL-12 p35 and EBI3 subunits, respectively. IL-35 binds to the homodimeric receptors, IL-12 R beta2 and gp130, as well as to the IL-12 R beta2-gp130 receptor heterodimer (4). The expression pattern of IL-35 is thought to differ between mouse and humans (5). In mouse regulatory T cells, both subunits of IL-35 are constitutively expressed and the mature IL-35 is secreted. In humans, IL-12 p35 is the only subunit constitutively expressed in regulatory T cells. Immune activation can induce EBI3 expression and IL-35 secretion in human effector T cells (6-8). IL-35 is also expressed and secreted in human placental trophoblasts (1, 9). In both human and mouse IL-35 has been shown to suppress effector T cell proliferation, inhibit Th17 cell development, and promote the conversion of T cells and B cells into regulatory T and B cells, respectively (1, 4, 8, 10, 11). IL-35 is thought to be involved in infectious tolerance and inflammatory cytokine-mediated autoimmune disorders (1, 3, 5, 12). Serum levels of IL-35 are associated with acute graft-versus-host disease following hematopoietic stem cell transplantation (13, 14). IL-35 also functions as a regulator of tumor growth (2, 12, 15).
References
- Collison, L.W. and D.A. Vignali (2008) Immunol. Rev. 226:248.
- Wang, Z. et al. (2013) J. Immunol. 190:2415.
- Choi, J. et al. (2015) Clin. Rev. Allergy. Immunol.
- Collison, L.W. et al. (2012) Nat. Immunol. 13:290.
- Ning-Wei, Z. (2010) Rev. Med. Chil. 138:758.
- Bardel, E. et al. (2008) J. Immunol. 181:6898.
- Guttek, K. and D. Reinhold (2013) Cytokine 64:46.
- Collison, L.W. et al. (2007) Nature 450:566.
- Mao, H. et al. (2013) Hum. Immunol. 74:872.
- Wang, R.X. et al. (2014) Nat. Med. 20:633.
- Niedbala, W. et al. (2007) Eur. J. Immunol. 37:3021.
- Collison, L.W. et al. (2010) Nat. Immunol. 11:1093.
- Liu, Y. et al. (2014) Leukemia. [Epub ahead of print; PMID: 25363669].
- Zhang, X. et al. (2014) Ann. Hematol. [Epub ahead of print; PMID: 25512184].
- Long, J. et al. (2013) Biochem. Biophys. Res. Commun. 430:364.
Long Name
Alternate Names
Gene Symbol
Additional IL-35 Products
Product Documents for Recombinant Human IL-35 Fc Chimera Protein, CF
Product Specific Notices for Recombinant Human IL-35 Fc Chimera Protein, CF
For research use only