Recombinant Human Sonic Hedgehog/Shh Protein, High Activity
R&D Systems, part of Bio-Techne | Catalog # 8908-SH

Key Product Details
Source
Accession #
Structure / Form
Conjugate
Applications
Product Specifications
Source
Cys24-Gly197
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
SDS-PAGE
Activity
The ED50 for this effect is typically 6-36 ng/mL.
Reviewed Applications
Read 1 review rated 4 using 8908-SH in the following applications:
Scientific Data Images for Recombinant Human Sonic Hedgehog/Shh Protein, High Activity
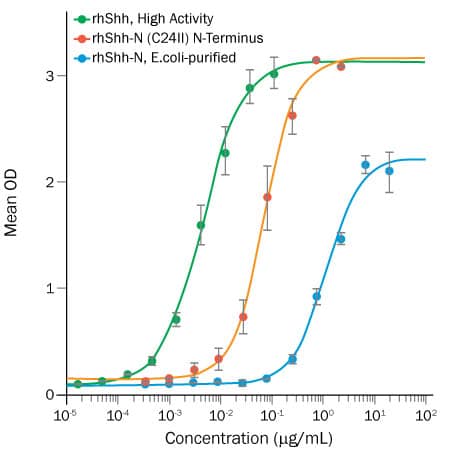
Enhanced Activity of Human Cell-expressed Sonic Hedgehog (Shh) Protein.
Recombinant Human Shh proteins induce alkaline phosphatase production by mesenchymal stem cells. High Activity Shh (green), purified from HEK293 cells and containing the correct post-translational modifications (cholesterol and fatty acids), is over 14-fold more active than E. coli-purified Recombinant Human Shh-N (C24II) N-Terminus (Catalog # 1845-SH; red line), and over 250-fold more active than E. coli-purified Recombinant Human Shh-N (Catalog # 1314-SH; blue line).Enhanced Activity of Human Cell-expressed Sonic Hedgehog (Shh) Protein.
1 μg/lane of Recombinant Human Sonic Hedgehog/Shh Protein was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by silver staining, showing 18-24 kDa bands.Post-translational Modification Analysis of Naturally-modified Recombinant Human Sonic Hedgehog (Shh) Protein.
LC/ESI-MS analysis of Recombinant Human (rh)SHH Protein, High Activity shows major peaks at 20119.3, 20145.2, and 20171.6 Da, suggesting that recombinant human SHH molecules are dual-modified with cholesterol at C-terminus, and fatty acids (lauric acid, myristic acid, and palmitic acid) at the N-terminus. The minor peaks at 19776 Da corresponds to rhSHH with only fatty acid modification.Formulation, Preparation and Storage
Carrier Free
What does CF mean?CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
What formulation is right for me?In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
Carrier: 8908-SH
| Formulation | Supplied as a 0.2 μm filtered solution in MES, NaCl and CHAPS with BSA as a carrier protein. |
| Shipping | The product is shipped with dry ice or equivalent. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Carrier Free: 8908-SH/CF
| Formulation | Supplied as a 0.2 μm filtered solution in MES, NaCl and CHAPS. |
| Shipping | The product is shipped with dry ice or equivalent. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: Sonic Hedgehog/Shh
References
- Briscoe, J. and P.P. Therond (2013) Mol. Cell. Biol. 14:416.
- Aviles, E.C. et al. (2013) Front. Cell. Neurosci. 7:86.
- Xie, J. et al. (2013) OncoTargets Ther. 6:1425.
- Marigo, V. et al. (1995) Genomics 28:44.
- Zeng, X. et al. (2001) Nature 411:716.
- Feng, J. et al. (2004) Development 131:4357.
- Goetz, J.A. et al. (2006) J. Biol. Chem. 281:4087.
- Pepinsky, R.B. et al. (1998) J. Biol. Chem. 273:14037.
- Chen, M.-H. et al. (2004) Genes Dev. 18:641.
- Etheridge, L.A. et al. (2010) Development 137:133.
- Jakobs, P. et al. (2014) J. Cell Sci. 127:1726.
- Dierker, T. et al. (2009) J. Biol. Chem. 284:8013.
- Lewis, P.M. et al. (2001) Cell 105:599.
- Carpenter, D. et al. (1998) Proc. Natl. Acad. Sci. USA 95:13630.
- Filmus, J. and M. Capurro (2014) Matrix Biol. 35:248.
- Chuang, P.-T. and A.P. McMahon (1999) Nature 397:617.
Alternate Names
Gene Symbol
UniProt
Additional Sonic Hedgehog/Shh Products
Product Documents for Recombinant Human Sonic Hedgehog/Shh Protein, High Activity
Product Specific Notices for Recombinant Human Sonic Hedgehog/Shh Protein, High Activity
For research use only