Recombinant SARS-CoV-2 B.1.324.1 Spike RBD His Protein, CF
R&D Systems, part of Bio-Techne | Catalog # 10830-CV
E484K, S494P, N501Y

Key Product Details
Product Specifications
Source
Human embryonic kidney cell, HEK293-derived sars-cov-2 Spike RBD protein
Arg319-Phe541 (Glu484Lys, Ser494Pro, Asn501Tyr), with a C-terminal 6-His tag
Arg319-Phe541 (Glu484Lys, Ser494Pro, Asn501Tyr), with a C-terminal 6-His tag
Purity
>95%, by SDS-PAGE visualized with Silver Staining and quantitative densitometry by Coomassie® Blue Staining.
Endotoxin Level
<0.10 EU per 1 μg of the protein by the LAL method.
N-terminal Sequence Analysis
Arg319
Predicted Molecular Mass
26 kDa
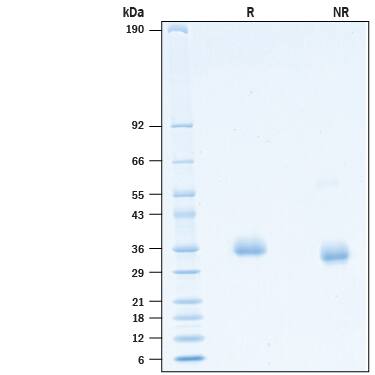
SDS-PAGE
33-40 kDa, under reducing conditions.
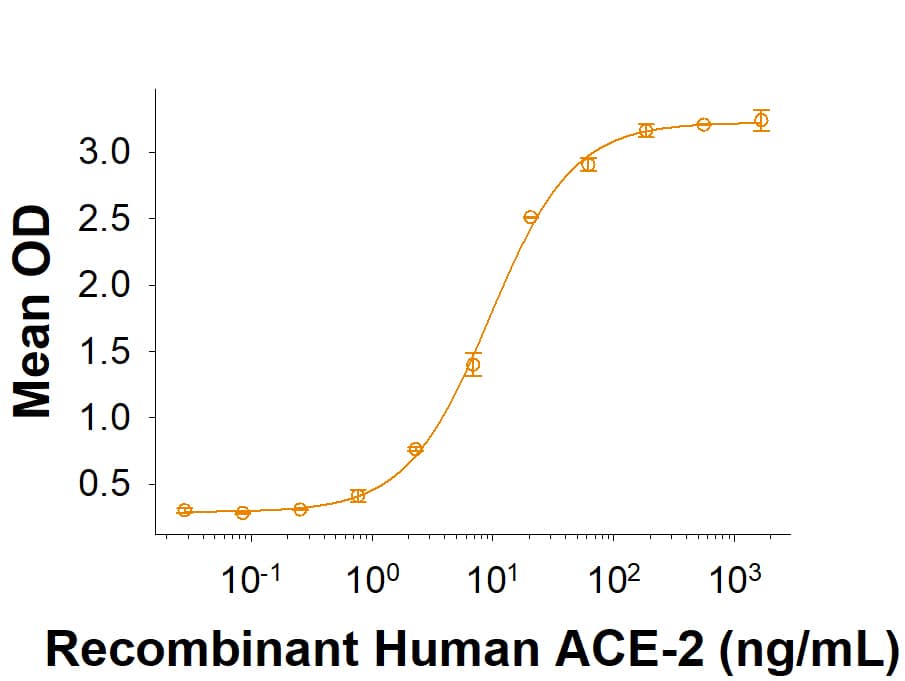
Activity
Measured by its binding ability in a functional ELISA with Recombinant
Human ACE-2 His-tag
(Catalog #
933-ZN).
Scientific Data Images for Recombinant SARS-CoV-2 B.1.324.1 Spike RBD His Protein, CF
Recombinant SARS-CoV-2 B.1.324.1 Spike RBD His-tag Protein Binding Activity.
Recombinant SARS-CoV-2 B.1.324.1 Spike RBD His-tag (Catalog # 10830-CV) binds Recombinant Human ACE-2 His-tag (933-ZN) in a functional ELISA.Recombinant SARS-CoV-2 B.1.324.1 Spike RBD His-tag Protein SDS-PAGE.
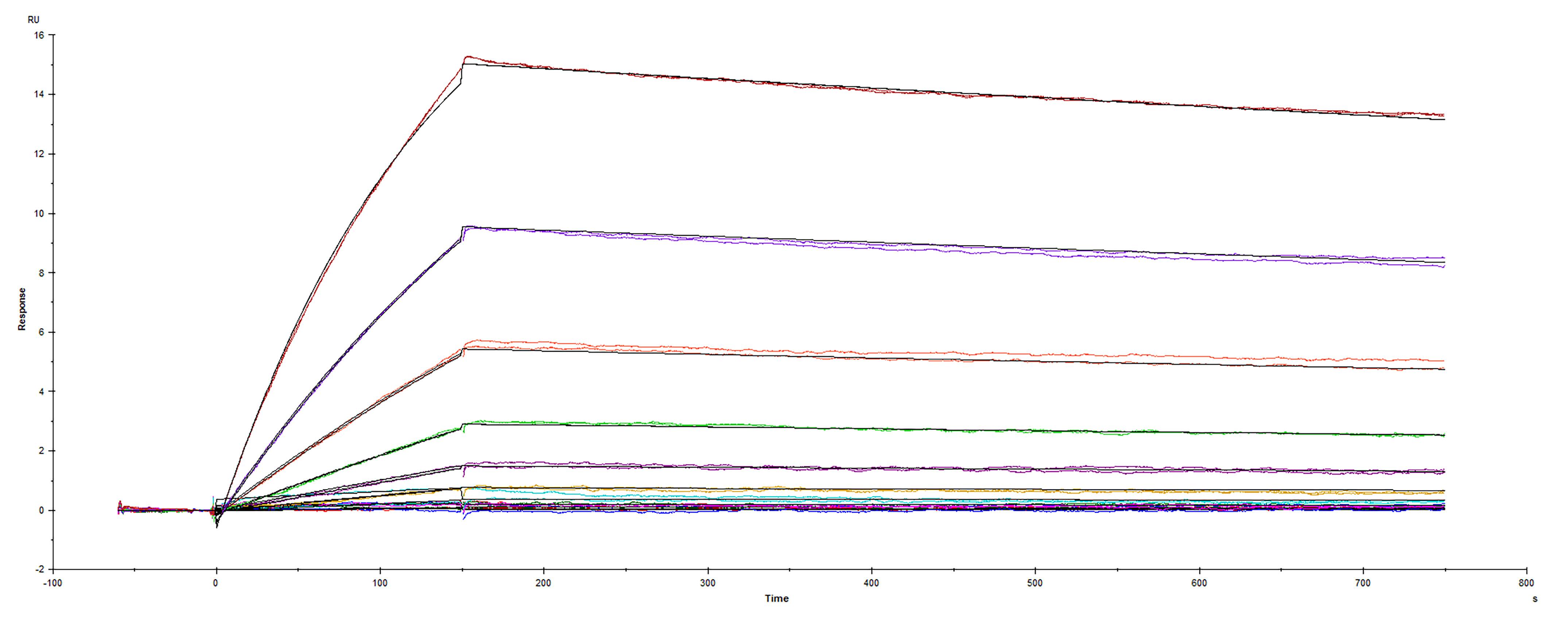
2 μg/lane of Recombinant SARS-CoV-2 B.1.324.1 Spike RBD His-tag Protein (Catalog # 10830-CV) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 33-40 kDa.Binding of ACE-2 to SARS-CoV-2 Spike RBD protein B.1.324.1 variant by surface plasmon resonance (SPR).
Recombinant SARS-CoV-2 Spike RBD protein B.1.324.1 variant His-tag was immobilized on a Biacore Sensor Chip CM5, and binding to recombinant human ACE-2 (933-ZN) was measured at a concentration range between 0.046 nM and 47.2 nM. The double-referenced sensorgram was fit to a 1:1 binding model to determine the binding kinetics and affinity, with an affinity constant of KD= 1.413 nM.Formulation, Preparation and Storage
10830-CV
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 500 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: Spike RBD
References
- Wu, F. et al. (2020) Nature 579:265.
- Tortorici, M.A. and D. Veesler (2019) Adv. Virus Res. 105:93.
- Bosch, B.J. et al. (2003) J. Virol. 77:8801.
- Belouzard, S. et al. (2009) Proc. Natl. Acad. Sci. 106:5871.
- Millet, J.K. and G.R. Whittaker (2015) Virus Res. 202:120.
- Li, W. et al. (2003) Nature 426:450.
- Wong, S.K. et al. (2004) J. Biol. Chem. 279:3197.
- Jiang, S. et al. (2020) Trends. Immunol. https://doi.org/10.1016/j.it.2020.03.007.
- Ortega, J.T. et al. (2020) EXCLI J. 19:410.
- Wrapp, D. et al. (2020) Science 367:1260.
- Tai, W. et al. (2020) Cell. Mol. Immunol. https://doi.org/10.1016/j.it.2020.03.007.1.
- Okba, N.M.A. et al. (2020). Emerg. Infect. Dis. https://doi.org/10.3201/eid2607.200841.
- Thornlow, B. et al. (2021) bioRxiv https://doi.org/10.1101/2021.04.05.438352.
- Wang, W.B. et al. (2021) bioRxiv https://doi.org/10.1101/2021.02.17.431566.
- Starr, T.N. et al. (2020) Cell. 182:1295.
- Zahradník, J. et al. (2021) bioRxiv https://doi.org/10.1101/2021.01.06.425392.
- Gu, H. et al. (2020) Science. 369:1603.
- Nonaka, C.K.V. et al. (2021) Emerg Infect Dis. https://doi.org/10.3201/eid2705.210191.
Long Name
Spike Receptor Binding Domain
Gene Symbol
S
UniProt
Additional Spike RBD Products
Product Documents for Recombinant SARS-CoV-2 B.1.324.1 Spike RBD His Protein, CF
Product Specific Notices for Recombinant SARS-CoV-2 B.1.324.1 Spike RBD His Protein, CF
For research use only
Loading...
Loading...
Loading...