BTLA Functions As a T Cell Co-inhibitory Receptor
Binding of HVEM to BTLA or CD160 Inhibits T Cell Activation
B and T lymphocyte attenuator (BTLA) is a co-inhibitory receptor belonging to the CD28 family that is structurally similar to CTLA-4 and PD-1.1 The extracellular domain of BTLA contains a single IgI-like domain followed by a transmembrane segment and a cytoplasmic tail that contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) and an immunoreceptor tyrosine-based switch motif (ITSM), which are involved in mediating inhibitory signaling.2-4 BTLA is expressed on B cells, T cells, natural killer (NK) cells, dendritic cells (DCs), and macrophages.5 While naïve CD4+ and CD8+ T cells express low levels of BTLA, BTLA expression is rapidly up-regulated following T cell activation.6, 7 In contrast to the other CD28 family receptors which interact with B7 family ligands, BTLA binds to herpesvirus entry mediator (HVEM), a member of the TNF receptor superfamily.8 HVEM is widely expressed on a variety of immune cell types, including dendritic cells, naive T and B cells, NK cells, monocytes, and neutrophils.6 Binding of HVEM to BTLA inhibits T cell and B cell activation, proliferation, and cytokine production, and serves as an inhibitory checkpoint regulating the accumulation of dendritic cells in lymphoid tissues.8-12 Additionally, in contrast to naïve effector T cells which down regulate HVEM expression upon activation, HVEM expression on regulatory T cells is up-regulated upon stimulation and its binding to BTLA, expressed on effector T cells, enhances their suppressive activity.7
Besides BTLA, HVEM also interacts with the immunoglobulin superfamily (IgSF) receptor, CD160, and the TNF superfamily proteins, Lymphotoxin alpha and LIGHT.13-15 While binding of HVEM to BTLA or CD160 delivers an inhibitory signal, HVEM also has the ability to stimulate the effector functions of immune cells on which it is expressed by binding to LIGHT or Lymphotoxin alpha.6, 13-15 As a result, HVEM has been described as a molecular switch regulating both co-stimulatory and co-inhibitory signaling.
In cancer, BTLA was reported to be expressed on CD8+ T cells isolated from the peripheral blood of melanoma patients and crosslinking of BTLA on these cells with HVEM on melanoma cells was shown to inhibit T cell expansion and IFN-gamma production.16 A second study demonstrated that BTLA up-regulation on tumor antigen-specific CD8+ T cells contributed to their dysfunction, and blockade of BTLA could at least partially restore CD8+ T cell proliferation and cytokine production.17 Additionally, this study demonstrated that blockade of both BTLA and PD-1 could synergistically improve the expansion and proliferation of tumor antigen-specific CD8+ T cells, when compared with anti-BTLA or anti-PD-1 treatment alone. The improvement in CD8+ T cell proliferation was even further enhanced with blockade of BTLA, PD-1, and TIM-3, suggesting that BTLA, PD-1, and TIM-3 all contribute to the dysfunction of tumor antigen-specific CD8+ T cells in melanoma. Based on these observations, BTLA is being investigated by immuno-oncology researchers and clinicians as a potential next generation target for cancer immunotherapy.
HVEM Regulates T Cell Co-inhibitory and Co-stimulatory Signaling

HVEM serves as a molecular switch regulating both T cell co-stimulatory and co-inhibitory signaling. HVEM is a TNF receptor superfamily protein that interacts with BTLA, CD160, LIGHT, and Lymphotoxin-alpha. While binding of HVEM to BTLA or CD160 produces a T cell co-inhibitory signal, HVEM binding to LIGHT or Lymphotoxin-alpha delivers a T cell co-stimulatory signal. The HVEM-BTLA interaction can also inhibit T cell functions by HVEM on regulatory T cells binding to T cell-expressed BTLA, which increases the suppressive activity of regulatory T cells.
Analysis of the Binding Activity of R&D Systems Recombinant Human BTLA Protein
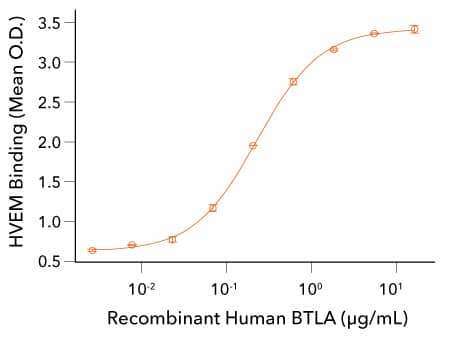
Recombinant Human BTLA Binds to HVEM. Recombinant Mouse HVEM/TNFRSF14 Fc Chimera (R&D Systems, Catalog # 2516-HV) was coated on a plate at 0.5 µg/mL and the indicated concentrations of Recombinant Human BTLA His tag (R&D Systems, Catalog # 9235-BT) were added. Recombinant Human BTLA bound with an ED50 of 0.1-0.5 µg/mL.
Assessment of the Binding Activity and Purity of R&D Systems Recombinant Cynomolgus Monkey CD160 Protein

Recombinant Cynomolgus Monkey CD160 Binds to HVEM. Recombinant Mouse HVEM/TNFRSF14 Fc Chimera (R&D Systems, Catalog # 2516-HV) was immobilized at 0.5 μg/mL and the indicated concentrations of Recombinant Cynomolgus Monkey CD160 Fc Chimera(R&D Systems, Catalog # 10197-CD) were added. Recombinant Cynomolgus Monkey CD160 bound with an ED50 of 1.5-9 ng/mL.

Assessment of the Purity of Recombinant Cynomolgus Monkey CD160 by CE-SDS on the MauriceTM System. The purity of Recombinant Cynomolgus Monkey CD160 Fc Chimera(R&D Systems, Catalog # 10197-CD) was assessed by capillary electrophoresis (CE)-SDS on the Maurice System under reducing (R) and non-reducing (NR) conditions and visualized in Compass for iCE software. The gel view is shown as an inset with the relative migration time (RMT) on the electropherogram shown on the far right-hand side of the gel.
-
Watanabe, N. et al. (2003) BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat. Immunol. 4:670. PMID: 12796776.
-
Compaan, D.M. et al. (2005) Attenuating lymphocyte activity: the crystal structure of the BTLA-HVEM complex. J. Biol. Chem. 280:39553. PMID: 16169851.
-
Gavrieli, M. et al. (2003) Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of B and T lymphocyte attenuator required for association with protein tyrosine phosphatases SHP-1 and SHP-2. Biochem. Biophys. Res. Commun. 312:1236. PMID: 14652006.
-
Chemnitz, J.M. et al. (2006) B and T lymphocyte attenuator-mediated signal transduction provides a potent inhibitory signal to primary human CD4 T cells that can be initiated by multiple phosphotyrosine motifs. J. Immunol. 176:6603. PMID: 16709818.
-
Hurchla, M.A. et al. (2005) B and T lymphocyte attenuator exhibits structural and expression polymorphisms and is highly induced in anergic CD4+ T cells. J. Immunol. 174:3377. PMID: 15749870.
-
Murphy, K.M. et al. (2006) Balancing co-stimulation and inhibition with BTLA and HVEM. Nat. Rev. Immunol. 6:671. PMID: 16932752.
-
Tao, R. et al. (2008) Regulatory T cell expression of herpesvirus entry mediator suppresses the function of B and T lymphocyte attenuator-positive effector T cells. J. Immunol. 180:6649. PMID: 18453584.
-
Sedy, J.R. et al. (2005) B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat. Immunol. 6:90. PMID: 15568026.
-
Gonzalez, L.C. et al. (2005) A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc. Natl. Acad. Sci. USA 102:1116. PMID: 15647361.
-
Vendel, A.C. et al. (2009) B and T lymphocyte attenuator regulates B cell receptor signaling by targeting Syk and BLNK. J. Immunol. 182:1509. PMID: 19155498.
-
Yu, X. et al. (2019) BTLA/HVEM signaling: milestones in research and role in chronic hepatitis B virus infection. Front. Immunol. 10:617. PMID: 30984188.
-
de Trez, C. et al. (2008) The inhibitory HVEM-BTLA pathway counter regulates lymphotoxin receptor signaling to achieve homeostasis of dendritic cells. J. Immunol. 180:238. PMID: 18097025.
-
Cai, G. et al. (2008) CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat. Immunol. 9:176. PMID: 18193050.
-
Harrop, J.A. et al. (1998) Herpesvirus entry mediator ligand (HVEM-L), a novel ligand for HVEM/TR2, stimulates proliferation of T cells and inhibits HT29 cell growth. J. Biol. Chem. 273:27548. PMID: 9765287.
-
Mauri, D.N. et al. (1998) LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity 8:21. PMID: 9462508.
-
Derre, L. et al. (2010) BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J. Clin. Invest. 120:157. PMID: 20038811.
-
Fourcade, J. et al. (2012) CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 72:887. PMID: 22205715.