The development of safe, effective immune cell therapies is a challenging road. Comparability, compliance, and product insights are essential in accelerating your R&D and clinical development programs. The key factors for bringing therapeutics to successful commercialization are:
- Lot-to-lot consistency, reproducibility, and scalability
- Understanding your therapy’s mechanism of action and its interactions in the tumor microenvironment
- Product characterization, defining the right critical quality attributes and the ability to measure them
- Accurate efficacy and toxicity testing for the validation of preclinical models
We understand the challenges you face in immune cell therapy development because we’ve been closely collaborating in the field for over 45 years. We commit to working in partnership with you, providing innovative solutions that support you throughout the immune cell therapy workflow.
Our pioneering technologies are designed to mitigate risks, boost reproducibility, and enable you to draw the meaningful conclusions you need to drive clinical advances.
Immune Cell Therapy Workflow
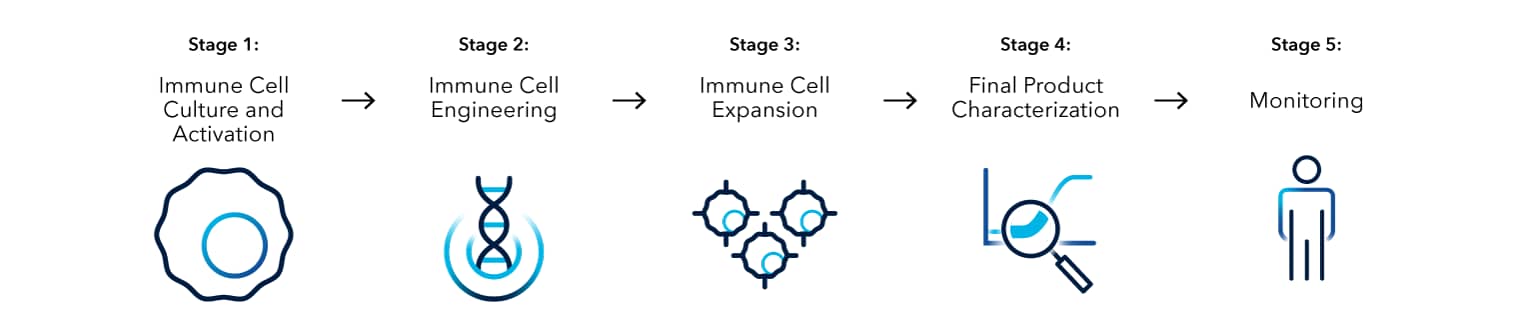
Immune Cell Culture and Activation
When looking to expand isolated leukocyte populations to scale, how can you ensure optimal growth conditions for consistent, reproducible lots? The best place to start is by using a reliable supplier that can provide a consistent supply of high-quality cell culture media, additives, and activators. Unlike many others, we manufacture 90% of our products in-house, letting us tightly control the supply chain to help you achieve lot consistency at scale.
Featured Solutions
Optimize Your Immune Cell Culture
Boost reproducibility in your cell culture with ExCellerate™ media. This highly-defined cell culture medium is optimized for immune cell culture, and is serum- and animal component-free for maximum lot consistency.
View Immune Cell Culture Media
Animal-Free RUO and GMP Cytokines
Accelerate your time to market by improving your culture expansion consistency. We manufacture our animal-free RUO cytokines and growth factors using the same process as our GMP cytokines to ensure they exhibit identical bioactivity to minimize process disruption during your transition to GMP.
Browse Animal-Free RUO and GMP Cytokines
GMP Antibodies
Unconjugated and unbound anti-CD3 and anti-CD28 offer the necessary flexibility to adapt to various cell therapy systems and ensure optimal phenotype.
Learn More About GMP Antibodies
Verify with Immunoassays
Deliver accurate, high-quality data consistently with our highly sensitive and specific immunoassay workflow solutions. Whether you’re quantifying cytokine activation with Ella™ or detecting the activation of intracellular signaling with our Simple Western™ systems, you can count on our systems to ensure the most accurate and reliable results.
Discover Immunoassay Solutions
QC Testing of GMP Proteins
Characterization of the quality of raw materials and reagents used during manufacturing is key to ensuring the safety and efficacy of your cell therapy products. Maurice™, an automated capillary electrophoresis instrument, streamlines the characterization of GMP proteins, saving you time, sample, and labor.
Resources
Immune Cell Engineering
Cell engineering is one of the most demanding and time-consuming steps of immune cell therapy development. From payload delivery to generating stable protein expression, the cell engineering stage can be a bottleneck. Save time, embed scalability into your process, and move forward with confidence with our cell engineering solutions.
Featured Solutions
Rapid Non-Viral Gene Delivery
Speed up gene delivery with the transposon-based TcBuster™. This non-viral system enables transgenes up to 10 kb to be quickly generated and electroporated into target cells – a valuable tool for both proof-of-concept and manufacturing process development.
Reliably Quantify CAR+ Cells
Easily evaluate your percentage of successfully engineered cells with Fluorokines™ - fluorescent-labeled recombinant proteins. Directly stain, detect and quantify CAR expression on your cells with high specificity by flow cytometry.
Cell Dispensing Made Easy
Dispense single cells and achieve optimal clonality with Namocell Single Cell Dispensers, enabling highly efficient and precise clone selection for cell engineering.
Discover Namocell Single Cell Dispensers
Automated Protein Expression Quantification
Easily quantify protein expression and confirm knock-out following gene editing with Simple Western – the only fully automated Western blot system on the market. Simple Western instruments combine capillary electrophoresis with sensitive immunodetection, allowing you to quantify target proteins in 3 hours, using just 3uL of sample.
Resources
Immune Cell Expansion
Obtaining robust cell expansion is important for downstream processing steps and meeting therapeutic dosing requirements. Scalability and lot consistency are the main challenges to overcome at this stage, and that’s why we provide high-quality bioreactors, media, and cytokines to help you generate large and consistent batches in every single run.
Featured Solutions
Quality Cytokines & Growth Factors
Increase safety, ensure bioactivity, and minimize variability with high quality Animal-Free RUO and GMP proteins. We ensure lot-to-lot consistency in both purity and bioactivity with our robust quality management system.
Browse Animal-Free and GMP Cytokines
Scale Up Your Process
Scale up and scale out with practicality, speed, and consistency via our ScaleReady partnership. G-Rex™ is a flexible, scalable gas-permeable cell culture bioreactor that delivers rapid cell expansion. G-Rex overcomes the limits of traditional plates and flasks with extended nutrient delivery and no added labor, pumps, mixing or shaking.
Optimize Your Immune Cell Cultures
Ensure optimal consistency in supply, formulation, quality, and lot consistency with our ExCellerate™ media. With formulations optimized for T cell or NK cell cultures, these media are both serum- and xeno-free.
Monitoring Cell Expansion
Our immunoassay workflow solutions enable sensitive and specific cell expansion analysis, whilst conserving precious samples. Using Simple Western™ systems, you can combine protein size or charge separation with a quantitative immunoassay readout, producing accurate, reproducible, and highly specific analytical-grade protein expression potency assays.
Discover Immunoassay Solutions
Resources
Final Product Characterization
Ensuring that your therapy meets all critical quality attributes (CQAs) requires assays and equipment with high specificity and sensitivity. Confirm that your finished product is functional, safe and consistent with customizable, flexible, and automated assay and analytics systems that provide you with the reliable, reproducible results you need.
Featured Solutions
Detect CARs with High Specificity
Discover a full suite of high-specificity Flow Cytometry antibodies, as well as Fluorokines™, fluorescent-labeled recombinant proteins, that enable highly specific detection of cell markers, CARs, immune checkpoint receptors, and more by flow cytometry.
Discover Flow Cytometry Reagents
Automate Your Cytokine Release Assays
Ella™ is a benchtop automated ELISA platform that delivers consistent, reproducible data with no manual steps. With Ella, you can perform highly specific and reliable potency assays of your final products, allowing you to detect key markers of cytotoxic activity such as IFN-γ.
Ella IFN-gamma Assay for QC Release Testing
Resources
Monitoring
It’s crucial to monitor engineered cells after infusion to validate the efficacy and safety of your cell therapy. Our monitoring solutions can track diverse metrics like cell trafficking, persistence, and activation, cytokine release syndrome (CRS), and disease biomarkers, to enhance your patient outcomes.
Featured Solutions
Verify Cell Therapy Delivery and Response with High Specificity
RNAscope ISH assays enable spatial detection of cell therapies in preclinical tissues and biopsy samples. The single cell and single molecule sensitivity enables robust analysis of therapeutic biodistribution, safety and efficacy assessment. Combining RNAscope ISH with Immunohistochemistry/Immunofluorescence allows detection of immune cells and their activation states within the tissue by visualizing cytokine expression in therapeutic and endogenous immune cells.
Learn More About RNAscope ISH Technology
High-Throughput Monitoring of the Immune Response
Our Luminex assays maximize multiplexing capacity and flexibility while maintaining assay specificity. These bead-based assays ensure robust and reproducible data, rapidly quantifying immune responses and cytokine release. Customize panels using the extensive mix-and-match menu, with up to 50 targets analyzed per sample. patient clinical immune monitoring
Decoding Tumor Complexity
By pairing the flexibility and specificity of Western blots with the analytical-grade quantitation of ELISA, Simple Western™ systems are ideal for shedding light on T-cell activation, exhaustion mechanisms, and the complexities of tumor microenvironments. For a more comprehensive understanding of cell heterogeneity, Single-Cell Westerns can analyze the tumor microenvironment at a single-cell level, revealing nuances in protein expression with unparalleled precision.
Resources
- Application Note: Preclinical CAR-T Cell Target Safety, Biodistribution, and Tumor Infiltration Analysis using the RNAscope ISH Assay
- Application Note: Keeping the Promise of Immuno-Oncology with Simple Western and Single-Cell Western
- Flyer: T-Cell Therapy Trafficking and Activation Analysis Using RNAscope in situ Hybridization
Supporting Your Cell Therapy from Benchtop to Clinic
Transitioning from research to the clinic can be challenging as you face more stringent qualification of raw materials. To help you move from RUO to GMP, we provide GMP cytokines and growth factors from our dedicated animal-free, GMP manufacturing facility. Each GMP product is directly comparable to our RUO animal-free version and is manufactured under ISO 9001 and 13485 Quality Management Systems, and to USP and European guidelines - so you can transition easily, with confidence in the supply and quality of your raw materials.
Our solutions can ease your transition from RUO to GMP:
- Scalable through commercialization
- Large lot sizes mean less bridging
- Full quality and regulatory support
- Drug Master Files available

ScaleReady™ Immune Cell Manufacturing Workflow
Our ScaleReady partnership accelerates immune cell therapy manufacturing, enabling high throughput parallel processing within a small footprint – efficiently producing more with less.
Custom Services for Immune Cell Therapy
Can’t find what you’re looking for or have specific requirements? We can help you optimize your process and minimize labor, waste, and risk. We bottle and package bulk reagents to fit your exact process requirements whether it’s research-grade or GMP‑grade.
Our custom immune cell therapy solutions include:
- GMP protein and antibody development
- Small molecules and chemistry services






