Immunocytochemistry (ICC) can be a very effective method for visualizing the localization and behavior of intracellular proteins, however the protocol for each ICC experiment should be optimized specifically to the cell being examined. Permeabilization, or the puncturing of the cell membrane, is an extremely important step in detecting intracellular antigens with a primary antibody because it allows entry through the cell membrane. Permeabilization is introduced after cells have been prepared with a fixative agent to initiate protein cross-linking, such as formaldehyde or ethanol. However, determining the amount of exposure to a permeabilization agent is crucial, seeing as a hard tissue organ may require a longer incubation over a soft tissue organ and treating your sample incorrectly will lead to unreliable results.
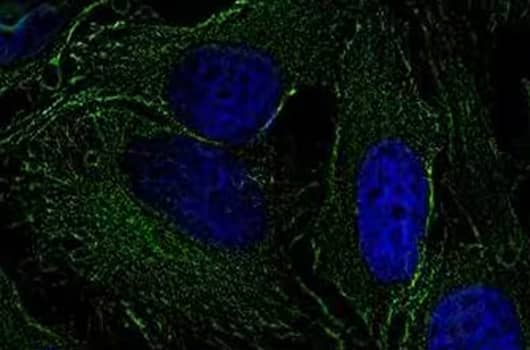
Tubulin Antibody (YL1/2) [NB600-506] - HeLa cells were fixed and permeabilized for 10 minutes using -20C MeOH. The cells were incubated with anti-Tubulin (YL1/2) at a 1:200 dilution overnight at 4C and detected with an anti-rat Dylight 488 (Green) at a 1:500 dilution. Nuclei were counterstained with DAPI (Blue). Cells were imaged using a 40X objective.
The two most common agents used to permeabilize the cell membrane are the detergents Triton-X 100 or Tween-20, with Tween-20 being the more gentle of the two. Triton-X 100 inserts a detergent monomer into the lipid membrane ultimately permeabilizing the membrane, whereas Tween-20 has a more renaturing effect on proteins and might improve antibody-antigen binding. Typically, the permeabilization agents are diluted into a phosphate buffer solution (PBS) in order to create enough volume required to incubate the entire sample. An example of different permeabilization methods using the same antibody can be observed with Chandra et al with their use of the LC3B antibody for ICC testing in macrophages. This group permeabilized with 0.2% Triton-X 100 in 1X PBS for 20 minutes. However, with the same LC3B antibody in C2C12 mouse myoblasts, Rashid et al used 0.5% Triton X-100 in 1x PBS for 5 minutes to optimize their results.
If the type, percentage and incubation period of your permeabilization agent are not optimized, you may end up with alternative results. For instance, an ICC image using a primary antibody after too long of a permeabilization may show an unusually high level of staining. On the other hand, an ICC experiment with too little permeabilization won’t allow the antibody to access the antigen, resulting in little to no staining at all. You can see that it is a fine balance between opening up the membrane enough to let only the primary antibody through. A large number of publications using Bio-Techne's antibodies in ICC are available to use as a resource for optimization, as well as reagents and a fully executed protocol for ICC. Request your copy of our ICC Handbook to learn more about permeabilization, protocols and troubleshooting tips.
-
Chandra, P. et al. () Mycobacterium tuberculosis Inhibits RAB7 Recruitment to Selectively Modulate Autophagy Flux in Macrophages PMID: 26541268.
-
Jamur, M.C. () Permeabilization of cell membranes PMID: 20012820.
-
Rashid, M.M. () Muscle LIM protein/CSRP3: a mechanosensor with a role in autophagy PMID: 27551448.
-
Koley, D. et al. () Triton X-100 concentration effects on membrane permeability of a single HeLa cell by scanning electrochemical microscopy (SECM) PMID: 20837548.
-
Zampieri, S. et al. () he use of Tween 20 in immunoblotting assays for the detection of autoantibodies in connective tissue diseases PMID: 10821942.