Three experienced industry leaders gathered for a virtual discussion around the remarkable progress and ongoing challenges in the development and manufacture of CAR-T and NK cell therapies.
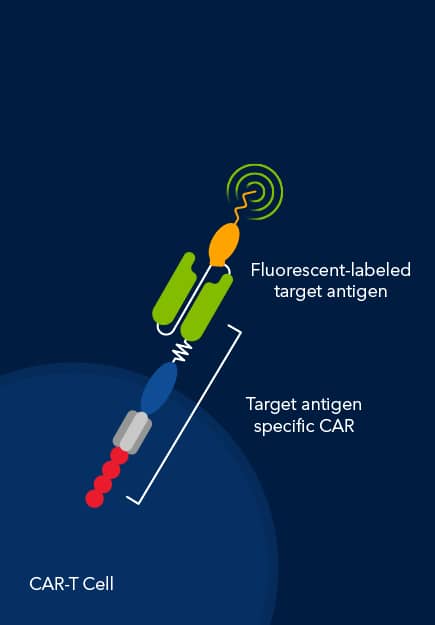
Dr. Tim Manning of Bio-Techne began by introducing Fluorokines™, a novel line of reagents designed to streamline the detection and analysis of CARs on modified T cells using flow cytometry. These fluorescent-labeled recombinant proteins target the most common CAR antigens, offering improved specificity and high lot-to-lot consistency. This kind of reliability is key to understanding of the amount of CAR that's expressed on your modified T cells.
Learn More About Fluorokines
Unpacking Development Challenges in CAR-T and CAR-NK Therapies
The panel then came together to unpick the hurdles and bottlenecks faced by developers in isolating and analyzing CAR expression during process development and production runs. Dr. Hemant Dhamne, discussed the need for specific assays for each individual product, multiplexing capabilities to evaluate other T cell markers, and the time-consuming nature of traditional flow cytometry protocols. All of these contribute to the challenges and delays in developing and producing CAR-based therapies.
Dr. Simone Steiner, who was involved in the development of the first FDA-approved CAR-T therapy, Kymriah, shared her perspective from the lens of large-scale commercial manufacturing. She emphasized the need for simple, reliable, and reproducible assays, as any variability or need for repetition could delay product release and jeopardize patient access.
“Flow cytometry is very individual; different operators may have different results, which is an absolute no go at large-scale.”
The discussion turned to current analytical methods, including ELISA, qPCR, and the ever-reliable flow cytometry. While each method has its strengths, they all have limitations and questions around reliability. Innovative tools and techniques are desperately needed to provide rapid, orthogonal data to quantify genome integration, and complement traditional analytics.

Future Directions and Innovations for CAR Therapies
So, what does the future hold? Where will the innovation we need come from? There was shared excitement for emerging technologies when the panel was asked for their wish list - unsurprisingly, closed systems and automation featured heavily. Also on the list was a move towards in vitro testing and analytics, in the form of live-cell imaging and organoid-based assays. Last on the list were cost-effective, non-viral gene delivery methods. Combined, these advancements have the potential to accelerate development timelines and provide deeper insights into the intricate mechanisms underlying CAR therapies, all in the name of getting therapies commercialized and to a broader patient population, faster.

But the challenges extend beyond the bench.
Navigating the regulatory landscape for these novel cell-based products remains a sizeable task. Simone emphasized the need for regulatory frameworks tailored to the unique nature of cell therapies, moving beyond the traditional approaches designed for biologics and not fit-for-purpose.
As the webinar drew to a close, a sense of optimism prevailed. While acknowledging the hurdles that lie ahead, the panelists' collective enthusiasm for the field was palpable. From extending CAR therapies to solid tumors, new indications and exploring allogeneic approaches, the future holds immense promise.
In the words of Dr. Hemant Dhamne, "I'm very fortunate to be part of this field and some of these my previous organizations, they have gone into treating patients and that really gives me the joy of contributing to this field".
It is this shared passion, coupled with collaborative efforts and innovative solutions, that will ultimately unlock the full potential of CAR-T and CAR-NK cell therapies, ushering in a new era of hope for cancer patients worldwide.
Audience Engagement: Key Questions and Insights
The webinar featured a host of questions from the audience too, it’s all available on demand, but here’s an overview the questions asked:
- Do you imagine an optimal range either in integrations per cell or CAR per cell on the surface? How does this parameter impact manufacturing processes and ultimately efficacy?
- What additional characterization tests are available for CAR products and what impact can this have on the final drug product? For example, the link between cell types and toxicities.
- What are the panel’s top tips or advice for successfully developing a CAR process? (spoiler alert: budget, budget, budget!)
- Do the Fluorokines work well with lower affinity CARs, or does it depend on the CAR?
- Which specific types of information can be obtained from flow cytometry with Fluorokines, which cannot be viewed by simpler methods used in QC testing and manufacturing?
- How are the latest tools and technologies helping to address some of the challenges still facing the CAR T and NK cell therapy sector, for example, response and relapse, toxicity, solid tumors and so forth?