Flow cytometry was developed to label and examine single cells with high throughput capacity using antibodies conjugated to fluorophores. The basic concept of flow cytometry is that a cell suspension is delivered as a single stream and is passed through a light source that uses detectors to generate data sets based off cellular properties. More specifically, the light emitted by fluorescently conjugated antibodies is channeled through selected filters to sort based off preset parameters or targets used. Flow cytometry is particularly useful in cell viability and proliferation assays, as well as diagnosing disease (particularly blood cancers).
When selecting a panel of fluorophores for your flow cytometry panel, it is important to follow a few basic guidelines. First, it is important to understand your flow cytometer. You should know the number and types of lasers present, their excitation capabilities and how to set your filters. Setting a filter too wide can lead to excessive background signal, leading to false positives. If no signal or weak fluorescence intensity is detected, be sure that all lasers are well aligned, as misalignment can result in weak signals. The use of calibration beads can help decipher instrument performance for each channel. Next, the desired fluorochromes are selected. Using the Spectra Viewer, the ability to choose robust dye colors is maximized, providing a visualization of fluorochromes that will fit both the needs of the experiment and instrument’s capabilities. As a rule, bright fluorophores should be conjugated to antibodies against low expressing cellular targets whereas fluorochromes of low brightness should be used for highly expressed proteins to avoid spillover and loss of resolution and sensitivity.
Flow cytometry is crucially dependent on the inclusion of appropriate controls, such as FMO (fluorescence-minus-one) and isotype controls, which enable spectral overlap and non-specific binding of reagents to be evaluated and considered. It is critical to use single-color controls for compensation when gating. Used in place of the primary antibody, isotype controls serve as a negative control.
The following is an example of a combination of fluorophores for a 6-color panel; FITC/Alexa Fluor 488, PE, PerCP-Cy5.5, PE-Cy7, APC/Alexa Fluor 647, and APC-Cy7/APC-H7). If you are using an 8-color panel, you can use the above fluorophores and simply supplement with AmCyan and Brilliant Violet 421. It is important to be conscious of the potential false positives produced by using tandem dyes, due to their tendency to show degradation. Due to their size and inability to efficiently cross the plasma membrane, tandem dyes are only recommended for extracellular staining.
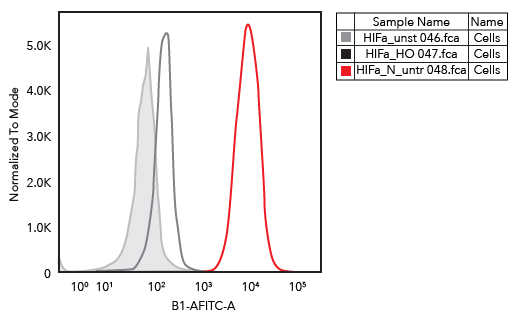
HIF-1 alpha Antibody (H1alpha67) [NB100-105] - Analysis using the Alexa Fluor (R) 488 conjugate of NB100-105. Staining of HIF-1 alpha in H929 cells using HIF-1 alpha antibody.
Our has an extensive list of conjugated antibodies available to meet your Flow Cytometry needs. To read a comprehensive overview of Flow Cytometry and how it works from start to finish, please visit our page on The Flow Cytometry Illustrated Assay or Troubleshooting Guide. To build your own sample Flow Cytometry panel, please take time to walk through our Panel Builder Tool. Bio-Techne offers flow cytometry validated primary antibodies backed by our guarantee.
-
Chattopadhyay, P.K. et al. () Brilliant violet fluorophores: a new class of ultrabright fluorescent compounds for immunofluorescence experiments PMID: 22489009.
-
Maecker, H.T. () Selecting fluorochrome conjugates for maximum sensitivity. PMID: 15536642.
-
Shaner, N.C. et al. () A guide to choosing fluorescent proteins. PMID: 16299475.