By Natalia Gurule, PhD
Breast Cancer is a Heterogeneous Disease
Breast cancer is the most frequently identified malignancy in women, accounting for 30% of diagnosed cases of cancer in women in the US annually.1 Although it is so prevalent, 70-80% of patients with early stage, non-metastatic disease experience cures. By contrast, the 5-year survival rate for metastatic breast cancer is 29%.2 Molecularly, breast cancer can be separated into 5 subtypes: Luminal A, Luminal B, Luminal B-like, HER2+, and Triple-negative (TNBC). TNBC is given its name because it is negative for the estrogen receptor, progesterone receptor, and HER2. Treatment strategies for breast cancer patients often differ based on the molecular subtype of their disease. In addition to traditional treatment modalities such as surgery, radiation, and chemotherapy, there has been an increase in therapies that target specific vulnerabilities unique to the cancer cell. These include endocrine therapy for hormone receptor positive disease, anti-HER therapies for HER2+ disease, poly(ADP-ribose) polymerase (PARP) inhibitors for patients carrying BRCA mutations, and immunotherapy. Despite there being multiple oncogene specific treatment options for patients with the other 4 molecular subtypes, patients with TNBC have limited targeted therapy options. TNBC patients develop resistance to chemotherapy at a frequency of 30-50% and often develop metastatic disease within 3-5 years of initial diagnosis. Thus, identifying vulnerabilities that can be specifically targeted in TNBCs is important because such treatments have the potential to significantly improve outcomes in these patients.
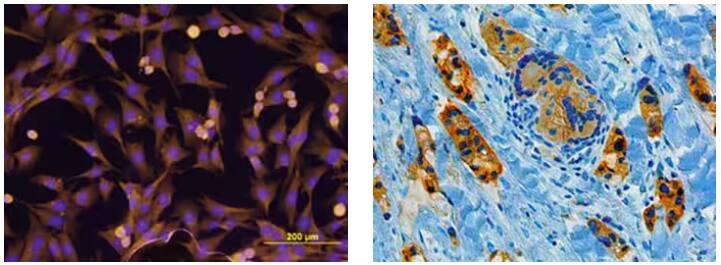
|
(Left) Immunocytochemistry analysis of ErbB2/Her2 detected in MDA‑MB‑231 human breast cancer cell line by probing with Mouse Anti-Human ErbB2/Her2 Monoclonal Antibody (Catalog # MAB1129), followed by staining of cells with NorthernLights™ 557-conjugated Anti-Mouse IgG Secondary Antibody (yellow; Catalog # NL007), and nuclear counterstaining with DAPI (blue). (Right) Chromogenic immunohistochemical analysis showing BRCA1 detected in immersion fixed paraffin-embedded sections of human breast cancer tissue by probing with Mouse Anti-Human BRCA1 Monoclonal Antibody (Catalog # MAB22101) overnight. Tissue was then subjected to heat-induced epitope retrieval using Antigen Retrieval Reagent-Basic (Catalog # CTS013), followed by staining using an Anti-Mouse HRP-DAB Cell & Tissue Staining Kit (brown), and counterstaining with hematoxylin (blue). |
Identifying NPC1 as a Novel Cell Vulnerability in TNBC
Recently, a team of researchers from the University of Colorado identified cholesterol transport as a novel vulnerability in TNBC cells. Niemann- Pick type C1 (NPC1) is a lysosomal cholesterol transporter that is responsible for cholesterol efflux from the lysosome to the endoplasmic reticulum during cellular processing of cholesterol that is endocytosed from outside the cell. Historically, NPC1 has been studied in the context of a lysosomal storage disorder that is caused by loss-of-function mutations in the NPC1 gene. Patients with this disorder develop abnormal accumulation of cholesterol within the lysosome which causes defects in trafficking and dysfunctional lysosomal function.
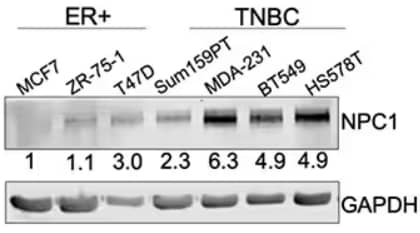
Western blot analysis on NPC1 expression in ER+ breast cancer cell lines compared to TNBC cell lines. Rabbit Anti-Human NPC1 Polyclonal Antibody (Catalog # NB400-148) was used to detect NPC1. Results showed higher expression of NPC1 in TNBC cell lines. Image obtained and cropped from O'Neill KI, Kuo LW, Williams MM, et al. NPC1 Confers Metabolic Flexibility in Triple Negative Breast Cancer. Cancers (Basel). 2022;14(14):3543. Published 2022 Jul 21. doi:10.3390/cancers14143543. Licensed under CC-BY.
Previous work by the team has demonstrated that TNBC cells have significantly lower expression of a micro-RNA called miR-200c, which is known to regulate epithelial to mesenchymal transition (EMT) by suppressing expression of mesenchymal genes and allowing cells to maintain a more epithelial like phenotype.3 The research team report that not only is NPC1 expression higher in TNBC, but restoration of miR200c expression in TNBC cells represses NPC1. Their work demonstrates that miR200c directly targets the 3’UTR of the NPC1 gene for micro-RNA mediated gene suppression. Decreases in proliferation and invasion were observed when NPC1 was silenced using shRNAs in TNBC cells, suggesting that NPC1 has a role in metastatic functions of cancer cells. Furthermore, the researchers show that loss of NPC1 in TNBC cells causes a reduction in oxygen consumption which is used to measure mitochondrial ATP production, and altered mitochondrial morphology that may account for the observed decreases in cancer cell growth and invasion. Taken together, this data suggests that NPC1 may promote an aggressive phenotype in TNBC cells through altered cellular metabolism. Therefore, targeting NPC1 in TNBC may serve as a potential novel treatment strategy to improve outcomes in breast cancer patients.
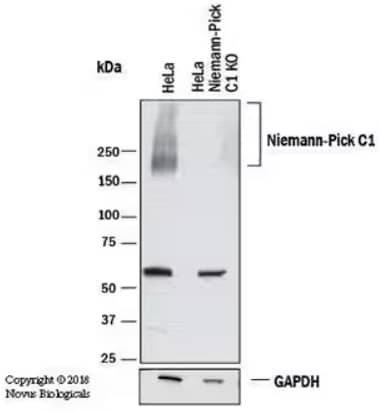
Genetic Strategies Validation. Western blot depicting lysates from HeLa parental cell line and NPC1 knockout (KO) cell line. The membrane was probed with Rabbit Anti-Human NPC1 Polyclonal Antibody (Catalog # NB400-148) followed by HRP-conjugated Anti-Rabbit IgG Secondary Antibody (Catalog # HAF008). A specific band for NPC1 was observed at ~ 240-260 kDa in the parental line but is not observed in the KO cell line. GAPDH was used as a loading control.
Note:
O’Neill et al. used Rabbit Anti-Niemann-Pick C1 Polyclonal Antibody (Catalog #NB400-148), Rabbit Anti-LDLR Polyclonal Antibody (Catalog #NBP1-06709), and Cultrex UltiMatrix Basement Membrane Extract (Catalog #BME001).
Natalia Gurule, PhD
Dr. Gurule is a postdoctoral fellow at National Jewish Health. She studies pathways that regulate inflammation upon infection in leukemia and myeloid dysplastic syndromes.
Research in Focus
O'Neill KI, Kuo LW, Williams MM, et al. NPC1 Confers Metabolic Flexibility in Triple Negative Breast Cancer. Cancers (Basel). 2022;14(14):3543. Published 2022 Jul 21. doi:10.3390/cancers14143543
Additional References
- Breastcancer.org
- Cancer.org
- Rogers TJ, Christenson JL, Greene LI, et al. Reversal of Triple-Negative Breast Cancer EMT by miR-200c Decreases Tryptophan Catabolism and a Program of Immunosuppression. Mol Cancer Res. 2019;17(1):30-41. doi:10.1158/1541-7786.MCR-18-0246