Synapsin-I, also called Synapsin 1/Syn1, is an ~80 kDa protein (predicted mol. wt. 74.1 kDa) which belongs to the Synapsin family (Synapsin I, Synapsin II, Synapsin III). Synapsins are the evolutionarily conserved phospho-proteins which are associated with the cytosolic side of the synaptic vesicles. They tether the vesicles to the actin cytoskeleton, thus forming a reserve pool. Synapsins I and II are generally found in mature synapses, whereas, Synapsin III is typically expressed in developing synapses with a relatively lower expression. Synapsins represents the most abundant of neuron-specific phospho-proteins, consisting of 9% of the total amount of all vesicle proteins in neurons wherein they play a critical role in the regulation of neurotransmitter release (Greengard et al., 1993). Phosphorylation at Ser-9 residue (phospho-Ser9 Synapsin I) results in dissociation of Synapsins I from synaptic vesicles which is important for synaptic vesicle neurotransmitter release. Synapsin I influences synaptic plasticity by regulating pre- and post-synaptic vesicular release. Mutations in Synapsin I have been linked to epilepsy, X-linked, with variable learning disabilities and behavior disorders (XELBD), which is a neurological disorder characterized by variable combinations of epilepsy, learning difficulties, macrocephaly, and aggressive behavior (Garcia et al., 2004).
Because of Synapsin I's characteristic subcellular localization, antibodies against this protein have been used as one of the most reliable markers for presynaptic vesicles. Our Synapsin I Antibodies have been cited in multiple publications from highly reputed research journals.
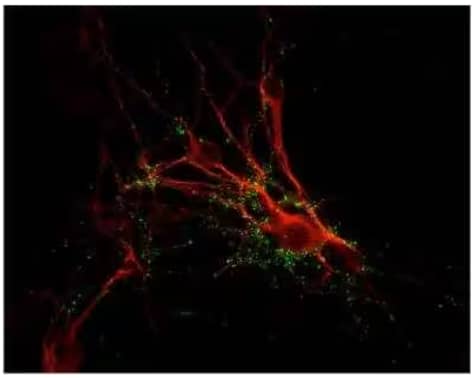
Synapsin I Antibody [NB300-104]
ICC/IF analysis showing punctate distribution
of Synapsin 1 protein (green) and MAP
protein (red) in cultured rat caudate neurons.
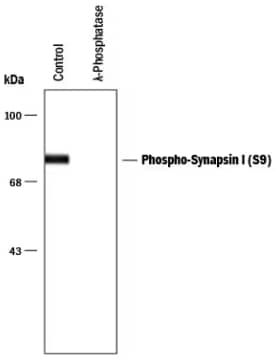
Synapsin I [p ser9] Antibody:
WB analysis of rat brain lysate which was
left un-treated (control) or was treated
with lambda-phosphatase: lambda PPase
Dr Antonio Pisani's team from the Laboratory of Neurophysiology and Synaptic Plasticity (University of Rome Tor Vergata) used our Synapsin I Antibody (Catalog # NB300-104) for WB analysis of synaptosomal preparations from cerebellar tissues of 14 day old mice. They used Synapsin I as well as post-synaptic density protein (PSD-95) as synaptic markers and established the localization of Torsin A/TOR1A in the cerebellar synaptosomal enrichments (Puglisi et al., 2013). A study published in Nature Communications by Dr Henley's lab from the University of Bristol UK cited the use of Synapsin I Antibody (Catalog # NB300-104) along with Bassoon as a presynaptic marker. Synapsin I antibody was used at 1:500 dilution for the immunocytochemistry/immunofluorescence analysis of paraformaldehyde fixed embryonic cortical and hippocampal neurons (isolated from Wistar rat's E18 embryos). The results from this study established SUMOylation-mediated regulation of Synapsin-Ia/Syn-Ia function and indicated an association of Syn-Ia's decreased SUMOylation with neurological disorders such as autism spectrum disorder/ASD and epilepsy (Tang et al., 2015).
Phophorylation of Synapsin-I at Ser-9 residue is critical because it leads to its dissociation from the synaptic vesicles. In a recent study published in Neuroscience, Dr Bozzi's team from University of Trento (Italy) used our Synapsin I [p Ser549] Antibody (Catalog # NB300-744) at 1:300 dilution for IHC on free-floating sections from brains of wild-type and Engrailed-2 knockout (En2−/−) mice, a model for autism spectrum disorders. The authors observed downregulated levels of SynI and its phosphorylation at Ser549/553 in hilus of mutant mice, before and after Morris water maze /MWM test (Provenzano et al., 2015).
Compiled by: Subhash Gangar
-
Garcia, C.C. et al. (2004) Identification of a mutation in synapsin I, a synaptic vesicle protein, in a family with epilepsy J Med Genet 41:183-186. PMID: 14985377.
-
Greengard, P. et al. (1993) Synaptic vesicle phosphoproteins and regulation of synaptic function Science 259:780-785. PMID: 8430330.
-
Provenzano, G. et al. (2015) Reduced phosphorylation of synapsin I in the hippocampus of Engrailed-2 knockout mice, a model for autism spectrum disorders Neuroscience 286:122-130. PMID: 25463523.
-
Puglisi, F. et al. (2013) Torsin A Localization in the Mouse Cerebellar Synaptic Circuitry PLoS One PMID: 23840813.
-
Tang, L.T. et al. (2015) SUMOylation of synapsin Ia maintains synaptic vesicle availability and is reduced in an autism mutation Nat Commun PMID: 26173895.