Intracellular Flow Cytometry Protocol Using Alcohol (Methanol)
The following flow cytometry protocol for staining intracellular molecules using alcohol to permeabilize cell membranes has been developed and optimized by Bio-Techne. For best results, use 1 x 106 cells per 100 μL of sample. Individual experimental designs for flow cytometry must be optimized, including antibody dilution and incubation time. For low cell density or poorly expressed intracellular targets, Single-Cell Westerns, such as Bio-Techne's Milo™ may be useful.
Please read the protocol in its entirety before starting.
Contents |
Materials
|
Sample Preparation
| Sample Type | Suggestions |
|---|---|
| Cells in Suspension | Suspended cells should be thoroughly washed with cold PBS to remove residual growth factors from cell culture media. After removing media remnants, use cells suspended in PBS to proceed with washing in Step 2. |
| Adherent Cells |
After removing media from adherent cells, add cold PBS to remove residual growth factors from cell culture media.
|
| Tissue |
To prepare tissues for flow cytometry, mechanical and/or enzymatic disaggregation is required.
|
Methods
|
|
Flow Cytometry BasicsWhy Use Flow Cytometry?In addition to phenotyping cells according to cellular morphology through forward and side scatter, antigen expression can be examined in various cell subtypes using flow cytometry. To assess intracellular antigen expression, cells must be fixed in suspension and then permeabilized before adding detection antibodies. It is important to use appropriate fixation and permeabilization reagents based upon the target and its subcellular location. Using antibodies for various intracellular molecules including phosphorylated signaling proteins and cytokines, flow cytometry is a powerful high-throughput tool. Several parameters can be measured simultaneously on millions of individual cells through the combination of different fluorescent probes in a multicolor panel. Permeabilization allows an antibody to pass freely across the membrane while maintaining the morphological characteristics required for phenotypic analysis and sorting. Due to their large size, tandem dyes are not recommended for intracellular staining as they have difficulty crossing the cell membrane to reach intracellular target proteins. When to Use Alcohols to PermeabilizeOrganic solvents (i.e. methanol/acetone) are more vigorous surfactants that can be used to permeabilize membranes by dissolving membrane lipids. Detergents on the other hand, create a porous membrane through mild permeation instead of dissolution of the membrane. Cold methanol is typically used to detect phosphorylated proteins and transcription factors. This flow cytometry protocol using alcohol as a permeabilization reagent is often required when detergents produce high background staining. It is important to note that methanol may decrease PE and APC conjugate signals. Therefore, cells should be permeabilized and washed prior to antibody addition to minimize methanol-fluorochromes interaction. Other Considerations
|
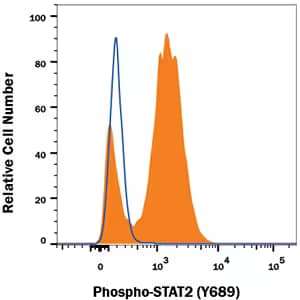
Detection of STAT2 in Daudi human cell line using methanol to permeate cells
Daudi human Burkitt's lymphoma cell line untreated (open histogram) or treated with 500 U/mL Recombinant Human IFN alpha A ( Catalog # 11100-1, filled histogram) for 20 minutes was stained with Rabbit Anti-Human Phospho-STAT2 (Y689) Monoclonal Antibody (Catalog # MAB2890), followed by Fluorescein-conjugated Anti-Rabbit IgG Secondary Antibody (Catalog # F0112). To facilitate intracellular staining, cells were fixed with Flow Cytometry Fixation Buffer (Catalog # FC004) and permeabilized with 90% methanol.