The First Bioactive Wnt1 Protein: Purification of Wnt1/sFRP Complexes
Abstract
Wnt proteins have been extremely difficult to purify in an active state due to their hydrophobic nature arising from post translational lipid modifications. Some Wnts, e.g. Wnt3a, Wnt5a/b, and Wnt8a, can be detected and purified in an active state from the conditioned media of CHO cells when they are overexpressed. Wnt1, however, seems to be primarily associated with the plasma membrane of Wnt producing cells and is not detected in the conditioned media of CHO cells overexpressing Wnt1. We discovered that sFRP expression, in Wnt1 producing cells, can liberate membrane tethered Wnt1 from the cell surface. Co-expression of sFRP1 or sFRP5 with Wnt1 in CHO cells results in detection of active Wnt1 in conditioned media. Co-culturing of sFRP1 or sFRP5 expressing CHO cells with Wnt1 expressing CHO cells also resulted in active Wnt1 in the conditioned media arguing against a mechanism of sFRP facilitating the ER/Golgi secretion pathway of Wnt1. Wnt1 and sFRP form a non-covalent complex which was purified in an aqueous purification procedure without using detergents, coating, or embedding methods for culturing stem cells, spheroids, and organoids.
Introduction
Wnt proteins are glycosylated and palmitoylated proteins that have proven extremely difficult to purify in an active state.1 These growth factors generally associate with the outer membrane of the Wnt producing cells resulting in limited secretion and signaling away from the Wnt production source. In 2003, Roel Nusse’s lab published a procedure to purify palmitoylated Wnt proteins using CHAPs detergent to maintain Wnts in a hydrophobic environment which proved essential for retaining Wnt activity.2,3 The structure of Xenopus laevis Wnt8 (XWnt8) bound to Fz8 receptor revealed that XWnt8 is palmitoylated on Serine 187, and that this lipid modification is critical for articulation with the Fz8 receptor.4 Secreted frizzled-related proteins (sFRPs) were originally described as Wnt inhibitory proteins,5-7 but were later shown to also enhance the diffusion of Wnt ligands and expand their signaling range.8-9 There are 5 sFRPs in the human and mouse genomes. They all show structural similarity to the Wnt binding cysteine rich domain (CRD) of Frizzled receptors. Unlike seven-pass transmembrane Frizzled receptors, sFRPs are secreted proteins that contain a netrin-like domain downstream of the CRD. We have demonstrated that sFRPs can serve as Wnt1 chaperone proteins and form a biologically active Wnt1/sFRP complex which can be further purified.
Methods and Results
Wnt1/sFRP1 Protein Complex
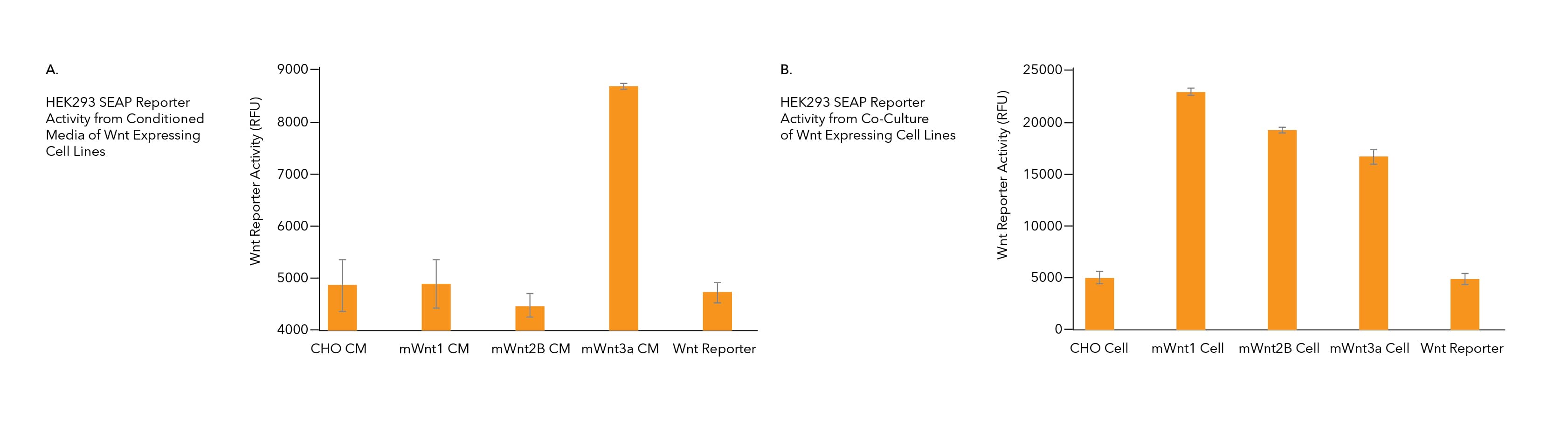
FIGURE 1. Wnt Proteins can be Either Secreted into Media or Associated with the Outer Membrane of the Wnt Producing Cells. A) Conditioned media (CM) from CHO cells expressing mWnt1 or mWnt2B were added to HEK293 Wnt reporter cells and did not induce Wnt reporter activity above conditioned media from wild type CHO cells or HEK293 Wnt reporter activity alone. The conditioned media from CHO cells expressing mWnt3a induced Wnt pathway activity when added to HEK293 Wnt reporter cells. B) Co-culture of mWnt1, mWnt2B, and mWnt3a producing CHO cells with HEK293 Wnt reporter cells results in Wnt pathway activation. Co-culture of wild type CHO cells with HEK293 Wnt reporter cells did not activate Wnt signaling.
| Treatment | Reporter Activity | |
|---|---|---|
| 1 | CHO cells (co-cultured with reporter cells) | - |
| 2 | CHO cell CM | - |
| 3 | CHO mWnt1 cells | + |
| 4 | CHO mWnt1 cell CM | - |
| 5 | CHO mFRP1 or msFRP5 cells | - |
| 6 | CHO mFRP1 or msFRP5 cell CM | - |
| 7 | CHO mWnt1 CM + CHO sFRP1/5 CM | - |
| 8 | CHO mWnt1 CM + CHO sFRP1/5 cell | - |
| 9 | CHO mWnt1 cell cultured with CHO sFRP1/5 CM overnight, then adding this CM to the Wnt reporter cells | + |
| 10 | CHO mWnt1/sFRP1/5 CM (Stable line expressing both mWnt1 and sFRP1 or sFRP5 in the same cell) | + |
| 11 | Adding sFRP1/5 protein to CHO mWnt1 expressing cells overnight and then adding this CM to Wnt reporter cells | + |
Table 1. sFRP1 or sFRP5 Liberates mWnt1 Protein from the Cell Surface when Wnt1 and sFRP1/5 are Expressed in the Same or Different Cells. Wild Type CHO cells co-cultured with Wnt Reporter HEK293 cells (Row 1) or conditioned media (CM) from wild type CHO cells cultured with Wnt Reporter HEK293 cells (Row 2) do not activate Wnt signaling. CHO cells expressing mWnt1, when co-cultured with Wnt reporter cells, activate Wnt signaling (Row 3). CM from mWnt1 expressing CHO cells (Row 4) or CM from msFRP1 or msFRP5 expressing CHO cells (Row 6), also do not activate Wnt Reporter activity. CHO cells expressing msFRP1 or msFRP5, when co-cultured with Wnt reporter cells, do not activate Wnt signaling (Row 5). CM from mWnt1 and msFRP1/5 expressing CHO cells, when combined and added to Wnt reporter cells, does not activate Wnt signaling (Row 7). CM from CHO cells expressing mWnt1, added to CHO cells expressing msFRP1 or msFRP5, does not result in Wnt reporter activation (Row 8). When CHO cell expressing mWnt1 are cultured with separate CHO cells expressing msFRP1 or msFRP5, this CM will activate Wnt reporter cells (Row 9). CM from stable lines expressing both mWnt1 and msFRP1 or msFRP5 will activate Wnt reporter cells (Row 10). If recombinant msFRP1 or msFRP5 protein is added to CHO cells expressing mWnt1, this CM will activate Wnt reporter cells (Row 11).
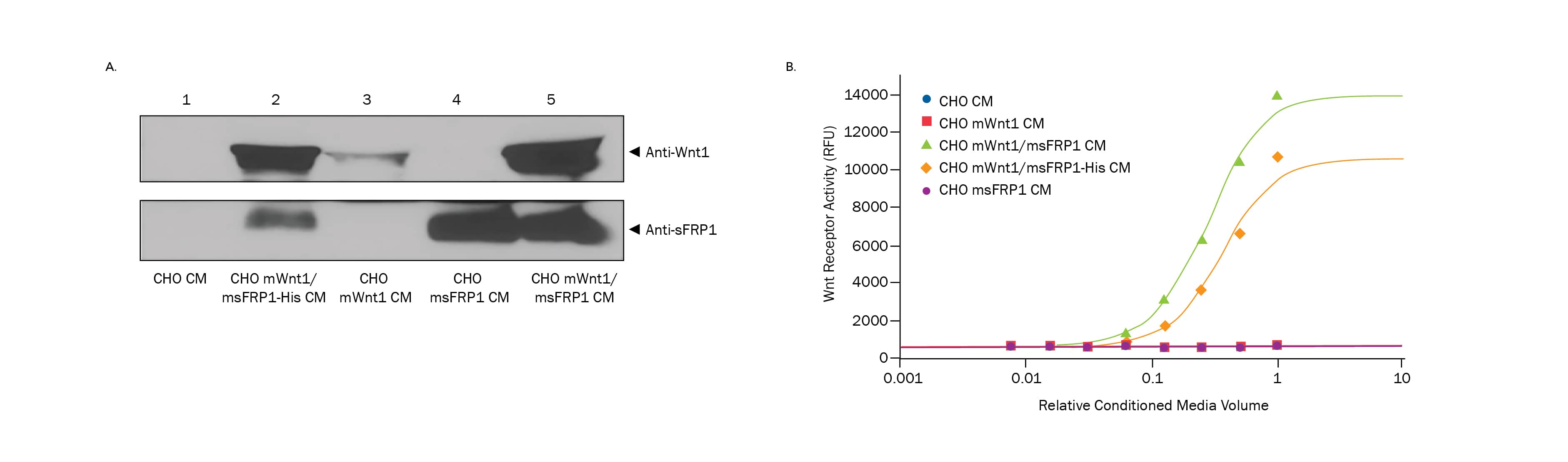
Figure 2. Co-Expression of sFRP1 in Cells Expressing Wnt1 Results in Bioactive Wnt1 in the Conditioned Media. A) Western blots of equal amounts of conditioned media (CM) show enhanced levels of mWnt1 in CHO cells expressing mWnt1/msFRP1-His (lane 2) and mWnt1/msFRP1 (lane 5) compared to mWnt1 levels in CHO control cell CM (lane 1), CHO mWnt1 CM (lane 3), and CHO msFRP1 (lane 4). msFRP1 Western blots show enhanced msFRP1 expression in CHO mWnt1/msFRP1-His CM (lane 2), CHO msFRP1 CM (lane 4), and CHO mWnt1/msFRP1 CM (lane 5) compared to CHO CM (lane 1) and CHO mWnt1 CM (lane 3). B) Conditioned media from CHO cells (blue circles), CHO mWnt1 (red squares), CHO mWnt1/msFRP1 (green triangles), CHO mWnt1/msFRP1-His (orange diamonds) and CHO msFRP1 (purple circles) were added to HEK293 TCF9-SEAP hFz4/hLRP5 Wnt reporter cells starting at the highest concentration of media and diluting 1:2. Only when CHO cells expressed mWnt1 and msFRP1 or msFRP1-His together was Wnt1 activity detected in the conditioned media. The conditioned media from CHO-s mWnt1/msFRP1 cells (green triangles) resulted in a 22-fold induction while the CHO mWnt1/msFRP1-His (orange diamonds) resulted in a 17-fold induction above background in this HEK293 TCF9-SEAP hFz4/hLRP5 Wnt reporter assay. Conditioned media from the other CHO lines did not induce activity in the HEK293 TCF9-SEAP hFz4/hLRP5 Wnt reporter cell line.
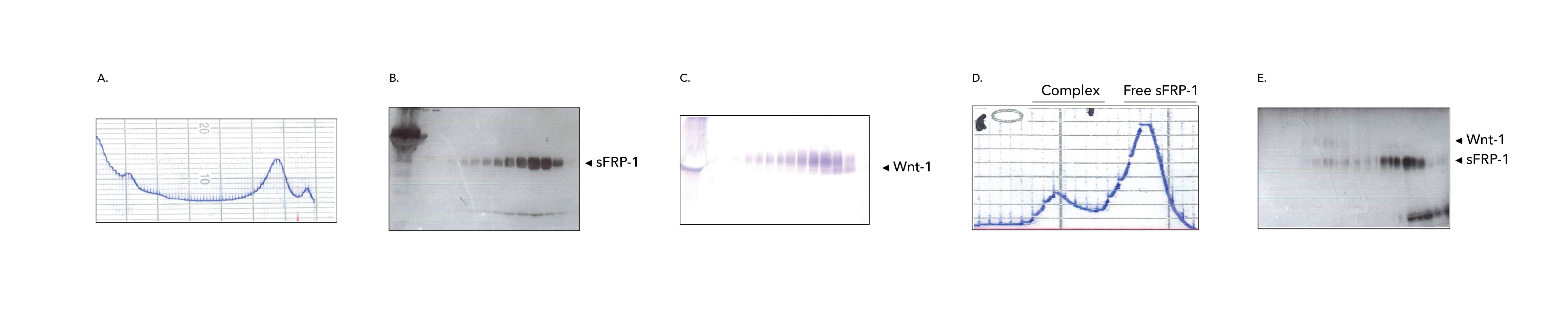
Figure 3. Purification of the Wnt1/sFRP Complex. A-C) Shows Wnt1 co-eluted with sFRP1 on the first chromatography. A) Conditioned media from mammalian cells co-expressing msFRP1 and mWnt1 were loaded and eluted from the first column. B) Elution fractions collected from the column were loaded onto 15% SDS-PAGE and stained with silver. The 37 kDa bands represents sFRP1. C) The same fractions in (B) were subjected to Western blot and probed with an antibody against Wnt1. The Wnt1 protein was detected as a ~52 kDa band that was present in the same fractions where sFRP1 was detected. D-E) Shows separation of sFRP1/ Wnt1 complex from free sFRP1 on the second chromatography step. D) Pool from the first column was loaded and eluted from the second column. E) Elution fractions collected from the second column were loaded onto 15% SDS-PAGE and stained with Coomassie blue. The lower band (37 kDa) represents sFRP1 and the upper band (52 kDa) represents Wnt1,
mWnt1/msFRP1 Complex on SDS-PAGE and Western Blot
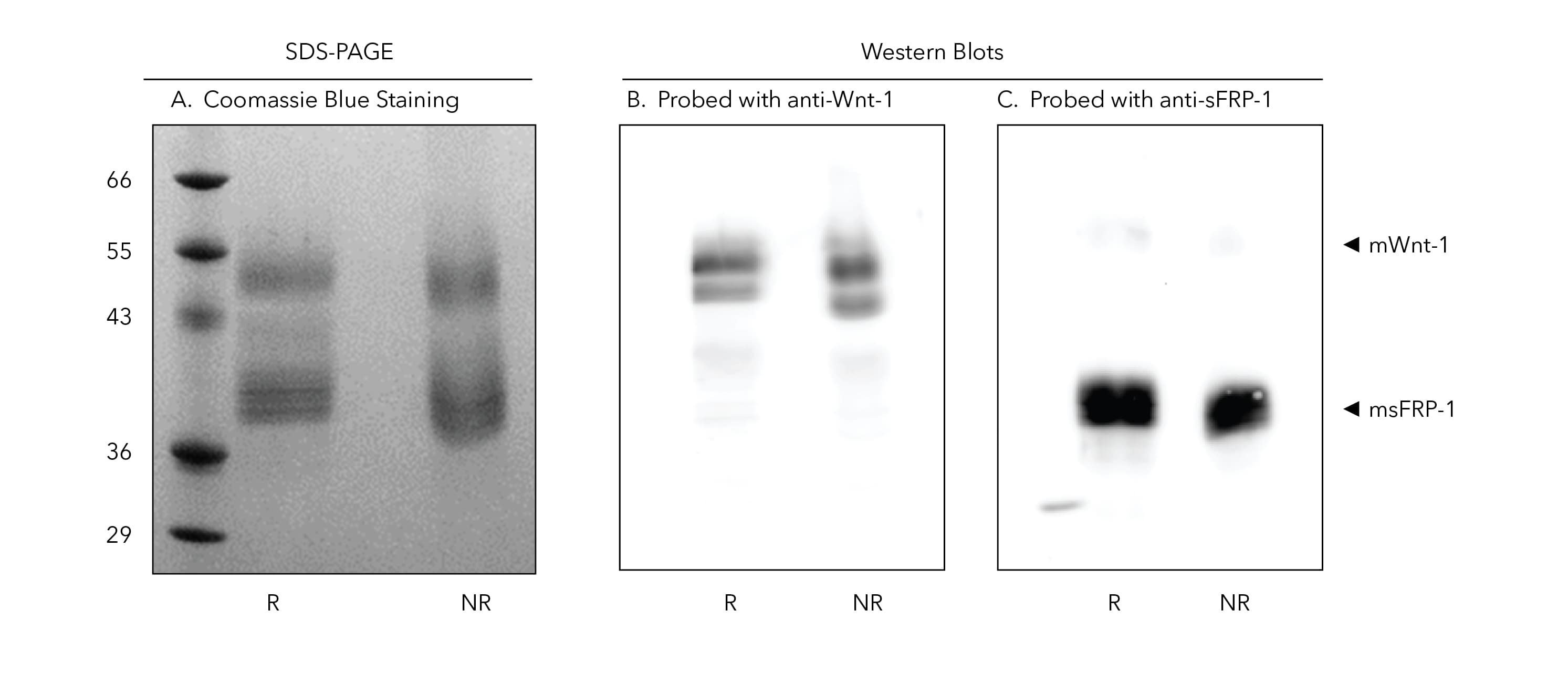
Figure 4. Both mWnt1 and msFRP1 Proteins are Detected in Coomassie Blue Staining and Western Blot when the Purified Recombinant mWnt1/msFRP1 Complex is Resolved on an SDS-PAGE Gel. A) 1 µg of mWnt1/msFRP1 was run on a 15% acrylamide gel under both reducing and non-reducing conditions and stained with Coomassie blue. B, C) 0.25 µg of mWnt1/msFRP1 was run on a 15% acrylamide gel under both reducing and non-reducing conditions and transferred to a PVDF membrane. Western blotting was performed with a goat anti-mWnt-1 antibody (1 µg/ml) (B) and a goat anti-hsFRP1 antibody (1 µg/ml) (C). A secondary antibody (donkey anti-goat HRP) antibody was used for detection at 1 µg/ml.
Figure 5. Purified mWnt1/msFRP1 Protein Complex Activates Wnt Signaling. Recombinant Mouse Wnt1/sFRP1 Complex (R&D Systems, Catalog #9765 WN) activates Wnt induced TCF reporter activity in HEK293 human embryonic kidney cells. The ED50 for this effect is 0.03–0.18 μg/mL.
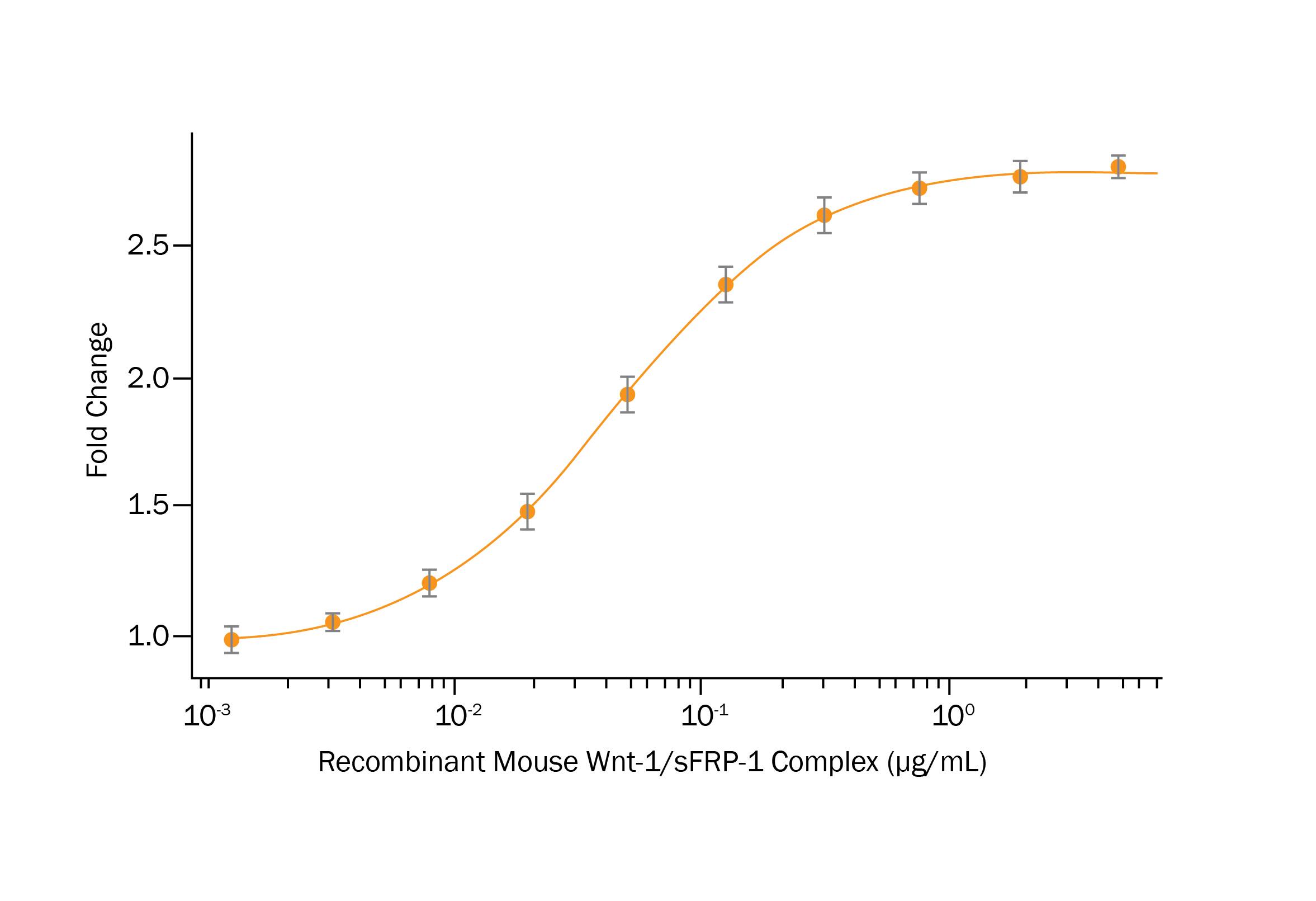
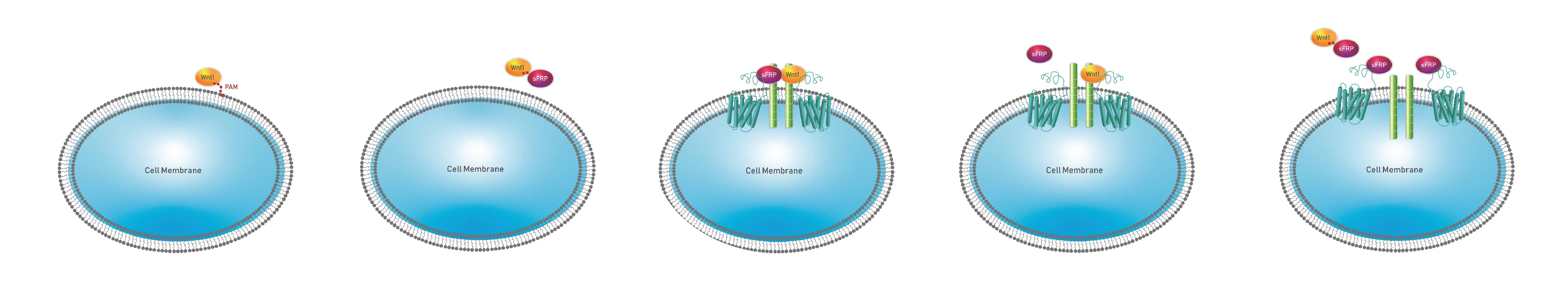
Figure 6. Model of Wnt1/sFRP Signaling. A) Wnt1 is associated with the plasma membrane due to the palmitoleic acid modification (PAM). B) sFRP binds to Wnt1 on the plasma membrane and presumably binds to the palmitoleic acid moiety on the Wnt1 protein resulting in a non-covalent heteromeric Wnt1/sFRP aqueous soluble complex. C) Wnt1/sFRP complexes associate with Frizzled receptors through binding of the sFRP portion of the Wnt1/sFRP complex to Frizzled receptors. D) sFRP dissociates from Frizzled receptors with Wnt1 remaining bound to other Frizzled receptors in Wnt receptor complexes. E) At high concentrations of free sFRP, free sFRPs bind to Frizzled receptors and inhibit binding of Wnt1/sFRP complexes to Frizzled receptors resulting in inhibition of Wnt signaling when sFRP concentrations are high.
Summary
- sFRP1 can act as a Wnt1 binding partner that binds to the palmitoleic acid moiety on mWnt1 shielding this lipid modification from the aqueous environment and therefore allowing membrane tethered mWnt1 to be purified in an active Wnt/sFRP complex.
- This strategy can be applied to many other membrane tethered Wnts. Active hWnt1/sFRP, mWnt2B/sFRP and hWnt6/sFRP complexes are presently being purified at R&D Systems.
Related Products
| Protein | Species | Catalog# |
|---|---|---|
| Wnt-1/sFRP1 Complex | H | 11160-WN |
| Wnt-1/sFRP1 Complex | M | 9765-WN |
| Wnt-2/sFRP-1 Complex | H | 11117-WN |
| Wnt-3/sFRP-1 Complex | M | 11200-WN |
- Willert, K. and R. Nusse (2012). Wnt proteins. Cold Spring Harb. Perspect. Biol. 4:a007864. doi:10.1101/cshperspect.a007864.
- Willert, K. et al. (2003). Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423:448. doi:10.1038/nature01611.
- Reya, T. et al. (2003). A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423:409. doi:10.1038/nature01593.
- Janda, C.Y. et al. (2012). Structural basis of Wnt recognition by Frizzled. Science 337:59. doi:10.1126/science.1222879.
- Rattner, A. et al. (1997). A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc. Natl. Acad. Sci. USA 94:2859.
- Wang, S. et al. (1997). Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell 88:757.
- Leyns, L. et al. (1997). Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell 88:747.
- Mii, Y. and M. Taira (2009). Secreted Frizzled-related proteins enhance the diffusion of Wnt ligands and expand their signaling range. Development 136:4083. doi: 10.1242/dev.032524.
- Esteve, P. et al. (2011). Secreted frizzled-related proteins are required for Wnt/β-catenin signaling activation in the vertebrate optic cup. Development 138:4179. doi:10.1242/dev.065839.