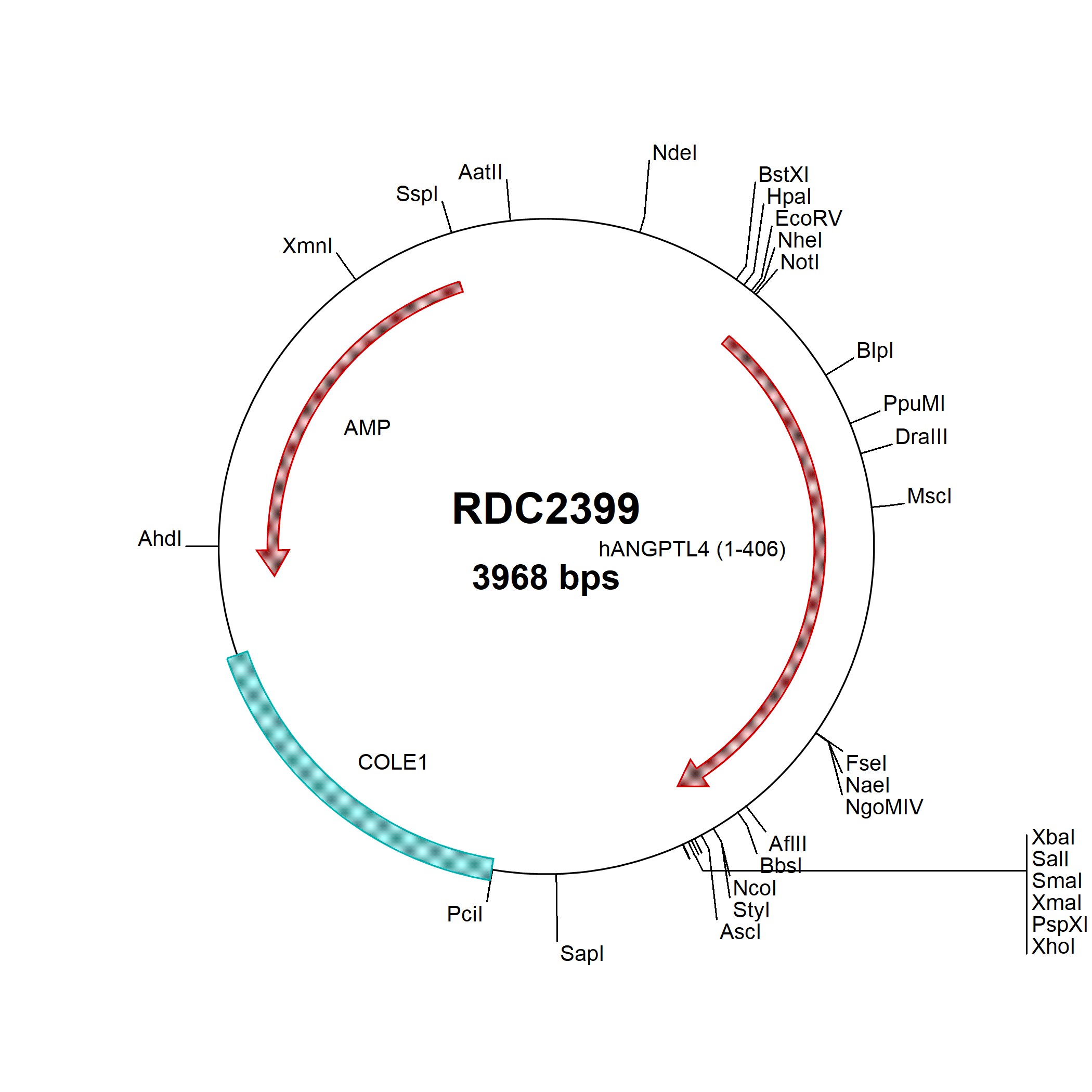
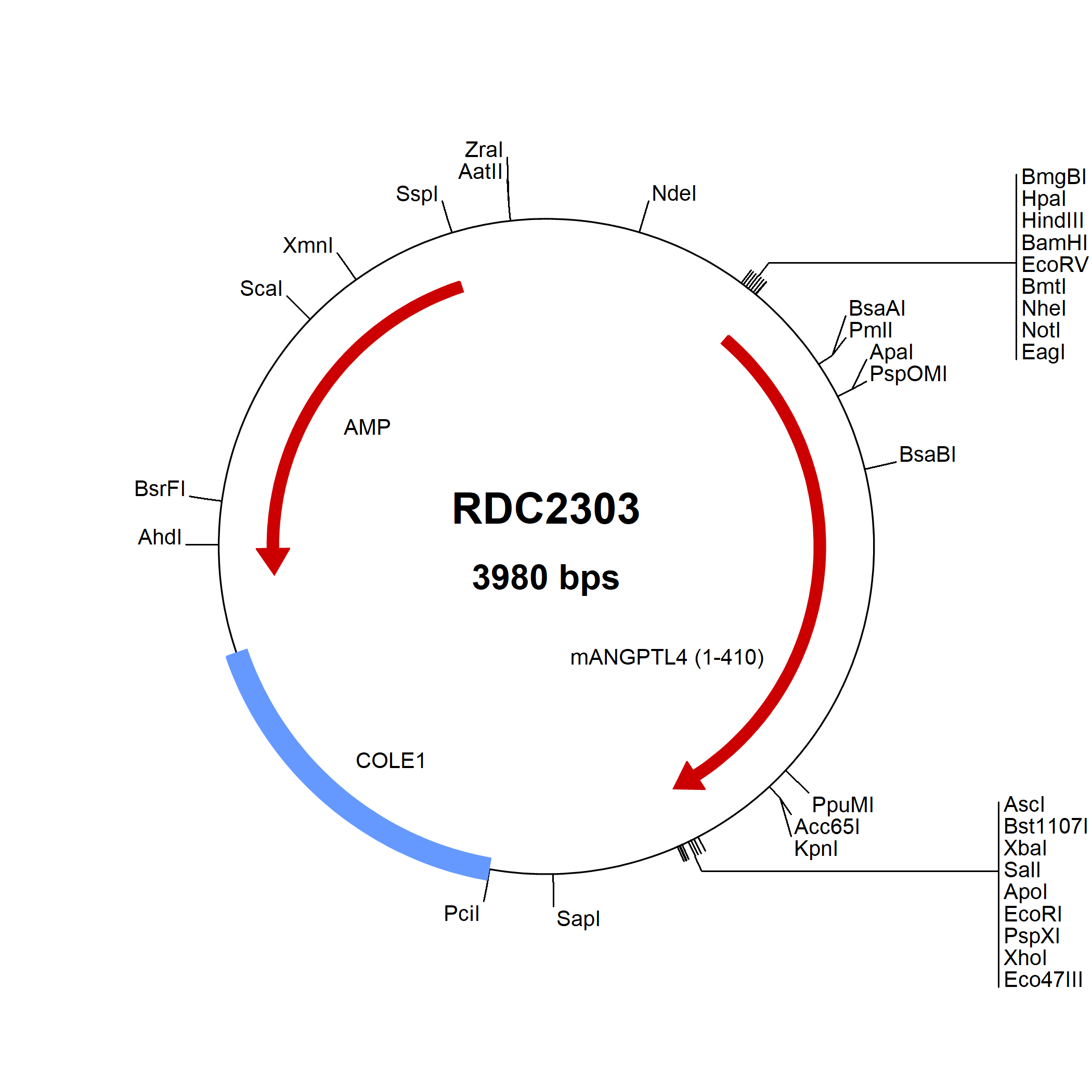
Angiopoietin-like Protein 4/ANGPTL4: cDNA Clones
Angiopoietin-like 4 (ANGPTL4), also known as FIAF, FARP, and PGAR, is a 55 kDa glycoprotein secreted by the liver and fat tissue. It contains an N-terminal coiled coil domain and a C-terminal fibrinogen-like domain which can be proteolytically separated. The coiled coil domain mediates the formation of variable-sized disulfide-linked oligomers. This domain directly inhibits lipoprotein lipase (LPL), resulting in increased circulating triglyceride levels.
ANGPTL4 circulates in association with HDL lipoproteins. Its expression in adipose tissue is induced by fasting and suppressed by feeding. In hypoxic areas, ANGPTL4 is induced in both vascular endothelial cells and tumor cells. The N-terminal fragment can function as an angiogenesis inhibitor. In contrast, the C-terminal fragment modulates cell adhesion through interactions with heparan sulfate proteoglycans, Integrins alpha 1 and beta 5, Vitronectin, and Fibronectin, thereby promoting keratinocyte migration and wound healing. ANGPTL4 additionally enhances the survival of hematopoietic and mesenchymal stem cells.
Products:
2 results for "Angiopoietin-like Protein 4/ANGPTL4 cDNA Clones" in Products
2 results for "Angiopoietin-like Protein 4/ANGPTL4 cDNA Clones" in Products
Angiopoietin-like Protein 4/ANGPTL4: cDNA Clones
Angiopoietin-like 4 (ANGPTL4), also known as FIAF, FARP, and PGAR, is a 55 kDa glycoprotein secreted by the liver and fat tissue. It contains an N-terminal coiled coil domain and a C-terminal fibrinogen-like domain which can be proteolytically separated. The coiled coil domain mediates the formation of variable-sized disulfide-linked oligomers. This domain directly inhibits lipoprotein lipase (LPL), resulting in increased circulating triglyceride levels.
ANGPTL4 circulates in association with HDL lipoproteins. Its expression in adipose tissue is induced by fasting and suppressed by feeding. In hypoxic areas, ANGPTL4 is induced in both vascular endothelial cells and tumor cells. The N-terminal fragment can function as an angiogenesis inhibitor. In contrast, the C-terminal fragment modulates cell adhesion through interactions with heparan sulfate proteoglycans, Integrins alpha 1 and beta 5, Vitronectin, and Fibronectin, thereby promoting keratinocyte migration and wound healing. ANGPTL4 additionally enhances the survival of hematopoietic and mesenchymal stem cells.
Products: