Heparanase/HPSE: Proteins and Enzymes
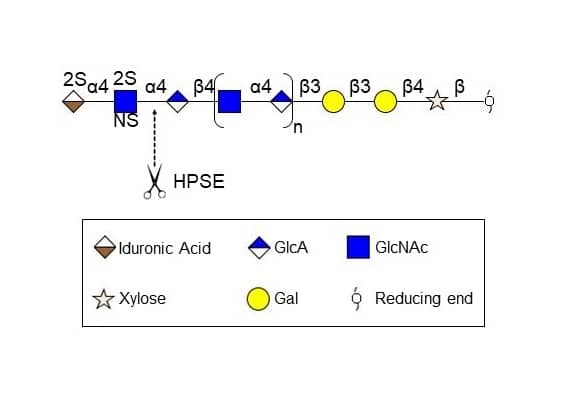
Heparanase (HPSE) selectively cleaves heparan sulfate at specific sites on heparan sulfate proteoglycans (HSPGs). The enzyme is synthesized as an inactive 65 kDa proenzyme that is secreted via the Golgi apparatus and associates with the cell membrane through interaction with HSPGs. It is then endocytosed and transferred to lysosomes where Cathepsin L activates it by removing an internal inhibitory peptide, forming a heterodimer composed of an 8 kDa and a 50 kDa subunit. Under certain stimuli, the active enzyme is transferred back to the cell surface, where it participates in extracellular matrix degradation and remodeling.
HPSE facilitates cell migration associated with metastasis, wound healing, and inflammation. An increase in its activity is associated with an increase in VEGF activity, which further enhances angiogenesis. HPSE also enhances the shedding of syndecans and increases endothelial invasion and angiogenesis in myelomas. It acts as a procoagulant by increasing the generation of activated Coagulation Factor X in the presence of Coagulation Factor III/Tissue Factor and activated Coagulation Factor VII. In addition, it increases cell adhesion to the extracellular matrix (ECM), independent of its enzymatic activity. HPSE is highly expressed in the placenta and spleen and weakly expressed in lymph nodes, thymus, peripheral blood leukocytes, bone marrow, endothelial cells, fetal liver, and tumor tissues.
Products:
2 results for "Heparanase/HPSE Proteins and Enzymes" in Products
2 results for "Heparanase/HPSE Proteins and Enzymes" in Products
Heparanase/HPSE: Proteins and Enzymes
Heparanase (HPSE) selectively cleaves heparan sulfate at specific sites on heparan sulfate proteoglycans (HSPGs). The enzyme is synthesized as an inactive 65 kDa proenzyme that is secreted via the Golgi apparatus and associates with the cell membrane through interaction with HSPGs. It is then endocytosed and transferred to lysosomes where Cathepsin L activates it by removing an internal inhibitory peptide, forming a heterodimer composed of an 8 kDa and a 50 kDa subunit. Under certain stimuli, the active enzyme is transferred back to the cell surface, where it participates in extracellular matrix degradation and remodeling.
HPSE facilitates cell migration associated with metastasis, wound healing, and inflammation. An increase in its activity is associated with an increase in VEGF activity, which further enhances angiogenesis. HPSE also enhances the shedding of syndecans and increases endothelial invasion and angiogenesis in myelomas. It acts as a procoagulant by increasing the generation of activated Coagulation Factor X in the presence of Coagulation Factor III/Tissue Factor and activated Coagulation Factor VII. In addition, it increases cell adhesion to the extracellular matrix (ECM), independent of its enzymatic activity. HPSE is highly expressed in the placenta and spleen and weakly expressed in lymph nodes, thymus, peripheral blood leukocytes, bone marrow, endothelial cells, fetal liver, and tumor tissues.
Products:
| Source: | CHO |
| Accession #: | AF144325 |
| Applications: | EnzAct |
| Source: | NS0 |
| Accession #: | Q6YGZ1 |
| Applications: | EnzAct |