By Jamshed Arslan, Pharm D, PhD
Introduction – Hippo kinase and its effectors Yap1 and Taz
Hippo signaling pathway may be regarded as a master regulator of organ growth. Hippo pathway is a sensor for cellular and tissue integrity that does not seem to have dedicated extracellular ligands or receptors. The downstream effectors of Hippo serine/threonine kinase signaling are two structurally and functionally similar proteins, Yap1 and Taz. These two proteins are transcriptional co-activators, which means that they themselves are unable to bind to the DNA. Rather, activated Yap1/Taz translocate to the nucleus and interact primarily with DNA binding transcription factors TEF1/Tead1-Tead4. It is mainly this Yap1/Taz-Tead protein complex that transcribes genes to control organ size and development.1,2 Activation of upstream Hippo signaling expels Yap1/Taz from nucleus and proteolytically degrades Yap1/Taz. Likewise, inactivating upstream Hippo signaling leads to tumorigenesis.3
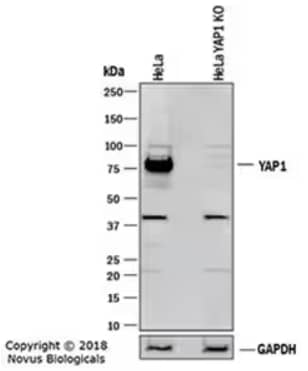
Western blot depicting lysates of HeLa parental cell line and YAP1 knockout (KO) HeLa cell line. PVDF membrane was probed for Rabbit Anti-Human YAP1 Polyclonal Antibody (NB110-58358) at 1:1000 followed by HRP-conjugated Secondary Antibody (HAF008). Specific band for YAP1 is present at approximately 75 kDa in the parental HeLa cell line, but is not detectable in the KO line.
Yap1/Taz in cell proliferation, migration, stemness, and survival
Yap1/Taz activation stimulates tissue repair and regeneration, but Yap1/Taz hyperactivation promotes cancer. The consequences of Yap1/Taz activation depend on the cell type and target genes. Hepatocytes exhibit proliferation upon activation of MCM3, MCM4, MCM6, and MCM10, but stemness and pluripotency is observed if OCT1/POU2F1, POU2F2, and SOX2 are activated. If the target genes of Yap1/Taz activation in hepatocytes are BIRC2, BIRC5, BCL2, and MCL1, cell survival is ensured. The cell fate decisions in hepatocytes, neural crest cells, neural retinal cells and many embryonic cells rely on Yap1-Taz-dependent activation of CDX2, GATA3, HΝF4A, JAG1, MYF5, MYF6, MYOG, and PROX1. Cell migration has been observed upon Yap1/Taz activation in endothelial cells, cardiac progenitor cells, prostate cancer and neural crest cells when target genes are CDH2, VIM, SNAI1, and SNAI2.1,2
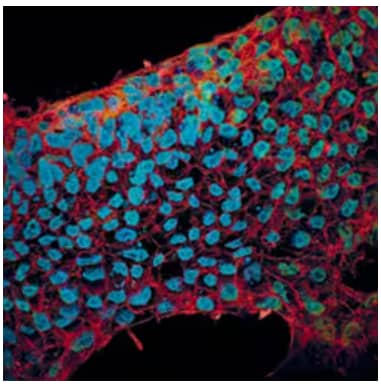
Immunocytochemical staining in BG01V human embryonic stem cells with 10 µg/mL Goat Anti-E‑Cadherin Polyclonal Antibody (AF648) and 10 µg/mL Mouse Anti-SOX2 Monoclonal Antibody (MAB2018), incubated for 3 hours at RT. Followed by secondary antibody staining for E‑Cadherin with NorthernLights™ 557-conjugated Secondary Antibody (NL001) (red) and for SOX2 with NorthernLights 493-conjugated Secondary Antibody (NL009) (green). Cells were counterstained with DAPI (blue).
Highlights from latest research
Researchers at Kindai University, Japan, recently discovered the molecular network underlying the proliferation of bone marrow-derived mesenchymal stem cells. The TGFβ-activated kinase 1 binds and stabilizes Yap1/Taz proteins to regulate mesenchymal stem cell proliferation. This observation has implications for stem cell transplantation.4
Reducing the nuclear localization of Yap1/Taz can be the underlying mechanism of cancer therapies. A collaboration between Chinese and German institutes found that a combination of Metformin and LW6 reduces cell proliferation and migration and induces cell death in pancreatic cancer cells. Metformin inhibits oxidative phosphorylation and LW6 inhibits malate dehydrogenase 2 enzyme. This combination increases phosphorylation of Yap1 and reduces nuclear localization of Yap1, thereby potentially treating pancreatic cancers.5
Researchers in Germany and South Korea shed light on the anti-angiogenic role of Yap1/Taz in the bone. Specifically, Yap1/Taz suppress the expression of HIF1-α target genes in the endothelial cells, thereby reducing vascular growth in an organ-specific and hypoxia-dependent manner.3
Unanswered questions
The regenerative role of Yap1/Taz activation has mainly been studied in mice and cultured cells. The behavior of primary human cells and organs upon Yap1/Taz activation is largely speculative. The aspects of transiently activating Yap1/Taz in human cells and human health are unknown. We do not know why Yap1/Taz are not triggered in organs with little or no regenerative potential. Answers to these questions will make Yap1/Taz at the epicenter of regenerative medicine.
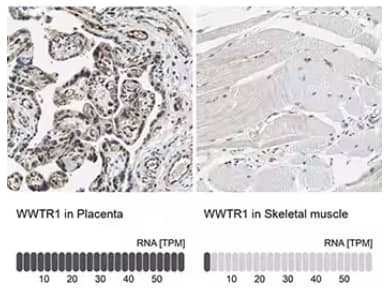
Immunohistochemistry-Paraffin staining in human placenta and skeletal muscle tissues with Rabbit anti-TAZ/WWTR1 Polyclonal Antibody (NBP1-85067). Below IHC-P images is the corresponding TAZ/WWTR1 RNA-seq data for the same tissues.

Jamshed Arslan, Pharm D, PhD
Dr Arslan is an Assistant Professor at Salim Habib University (formerly, Barrett Hodgson University), Pakistan.
His interest lies in neuropharmacology and preparing future pharmacists.
-
Boopathy, G., & Hong, W. (2019) Role of Hippo Pathway-YAP/TAZ Signaling in Angiogenesis. Frontiers in cell and developmental biology
-
Moya, I. M., & Halder, G. (2019) Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nature reviews. Molecular cell biology.
-
Sivaraj, K. K. et al. (2020) YAP1 and TAZ negatively control bone angiogenesis by limiting hypoxia-inducible factor signaling in endothelial cells eLife
-
Onodera, Y. et al. (2019) Transforming Growth Factor β-Activated Kinase 1 Regulates Mesenchymal Stem Cell Proliferation Through Stabilization of Yap1/Taz Proteins. Stem cells (Dayton, Ohio).
-
Zhang, X. et al. (2020) Metformin and LW6 impairs pancreatic cancer cells and reduces nuclear localization of YAP1. Journal of Cancer