Human Caspase-8 Antibody
R&D Systems, part of Bio-Techne | Catalog # MAB8135

Key Product Details
Validated by
Species Reactivity
Validated:
Cited:
Applications
Validated:
Cited:
Label
Antibody Source
Product Specifications
Immunogen
CGIPVETD
Accession # Q14790
Specificity
Clonality
Host
Isotype
Scientific Data Images for Human Caspase-8 Antibody
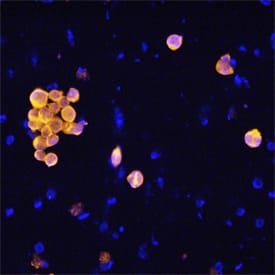
Caspase‑8 in Jurkat Human Cell Line.
Caspase-8 was detected in immersion fixed Jurkat human acute T cell leukemia cell line treated for 4 hours with staurosporin using Mouse Anti-Human Caspase-8 Polyclonal Antibody (Catalog # MAB8135) at 25 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Mouse IgG Secondary Antibody (yellow; Catalog # NL007) and counterstained with DAPI (blue). Specific staining was localized to cytoplasm. View our protocol for Fluorescent ICC Staining of Non-adherent Cells.Applications for Human Caspase-8 Antibody
Immunocytochemistry
Sample: Immersion fixed Jurkat human acute T cell leukemia cell line treated for 4 hours with staurosporin
Reviewed Applications
Read 1 review rated 5 using MAB8135 in the following applications:
Formulation, Preparation, and Storage
Purification
Reconstitution
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Caspase-8
Caspase-8 (Cysteine-aspartic acid protease 8/Casp8a; also named MCH5, FLICA and MACH alpha1) is a 28 kDa member of the peptidase C14A family of enzymes (1, 2, 3). It is widely expressed and is considered an initiating caspase for the apoptotic cascade (4). Caspase-8 acts on a wide variety of substrates, including procaspases-3, 4, 6, 7, 9 and 10, c-FLIPL and procaspase‑8 itself (1, 5, 6). Human procaspase-8a is a 54‑56 kDa, 479 amino acid (aa) protein (4, 7, 8, 9). It contains two N-terminal death domains (aa 1‑177), followed by a catalytic site that utilizes His317Gly318 plus Cys360. Normally, it is an inactive, cytosolic monomer (1, 10, 11). But following death-domain (DD) containing receptor oligomerization, Caspase-8 is recruited to the death-inducing signaling complex (DISC) that forms around the death domains of the oligomerized receptor (12). FADD/CAP-1 is recruited first, followed by procaspase-8/CAP-4 and, possibly, c-FLIPL and procaspase‑10 (12). The recruitment, or concentration, of procaspase-8 induces homodimerization. This act alone is sufficient for activation. However, the activity level is modest at best, and appears to be directed towards either itself, or c-FLIPL, which is known to form a functional heterodimer with procaspase-8 (5, 11). When directed towards itself, autocleavage occurs first between Asp374Ser375, generating a 43 kDa (p43) N-terminal (aa 1‑374) and an 11 kDa C‑terminal (aa 375‑479) fragment. The C‑terminus is further cleaved between Asp384Leu385 to generate a mature p10 subunit (aa 385‑479). The p43 subunit is next cleaved twice, once between Asp216Ser217, and again between Asp210Ser211 to generate a 26 kDa DD-containing prodomain (aa 1‑210) with an additional 18 kDa mature p18 subunit (aa 217‑374) (12). p18 and p10 noncovalently associate to form a 28 kDa heterodimer, which subsequently associates with another p18:p10 heterodimer to form an active, mature Caspase-8 molecule. This leaves the DISC to act on downstream apoptotic procaspases. In the event procaspase-8 comes to the DISC complexed with c‑FLIPL, c‑FLIPL will be cleaved by procaspase-8, generating a p43 fragment that is analogous to the Caspase-8 p43 subunit. This fragment, however, appears not to be an intermediate in a proteolytic cascade. Rather, it serves as a functional subunit, interacting with TRAF2 and activating NF kappaB. This may account for many of the nonapoptotic activities associated with Caspase-8 (5, 6, 13). Mature human and mouse Caspase-8a heterodimers are 73% aa identical (14).
References
-
Chowdhury, I. et al. (2008) Comp. Biochem. Physiol. B 151:10.
-
Boatright, K.M. & G.S. Salvesen (2003) Curr. Opin. Cell Biol. 15:725.
-
Launay, S. et al. (2005) Oncogene 24:5137.
-
Srinivasula, S.M. et al. (1996) Proc. Natl. Acad. Sci. USA 93:14486.
-
Hughes, M.A. et al. (2009) Mol. Cell 35:265.
-
Lamkanfi, M. et al. (2007) Cell Death Differ. 14:44.
-
Fernandes-Alnemri, T. et al. (1996) Proc. Natl. Acad. Sci. USA 93:7464.
-
Boldin, M.P. et al. (1996) Cell 85:803.
-
Muzio, M. et al. (1996) Cell 85:817.
-
Donepudi, M. et al. (2003) Mol. Cell 11:543.
-
Boatright, K.M. et al. (2003) Mol. Cell 11:529.
-
Golks, A. et al. (2006) Cell Death Differ. 13:489.
-
Scaffidi, C. et al. (1997) J. Biol. Chem. 272:26953.
-
Sakamaki, K. et al. (1998) Eur. J. Biochem. 253:399.
Alternate Names
Gene Symbol
UniProt
Additional Caspase-8 Products
Product Documents for Human Caspase-8 Antibody
Product Specific Notices for Human Caspase-8 Antibody
For research use only