Human CDX2 Antibody
R&D Systems, part of Bio-Techne | Catalog # MAB3665

Key Product Details
Validated by
Species Reactivity
Validated:
Cited:
Applications
Validated:
Cited:
Label
Antibody Source
Product Specifications
Immunogen
Asn54-Thr185
Accession # Q99626
Specificity
Clonality
Host
Isotype
Scientific Data Images for Human CDX2 Antibody
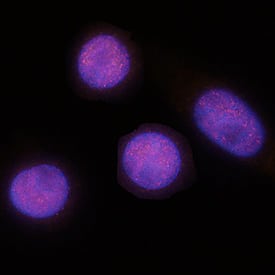
CDX2 in SW480 Human Cell Line.
CDX2 was detected in immersion fixed SW480 human colorectal adenocarcinoma cell line using Mouse Anti-Human CDX2 Monoclonal Antibody (Catalog # MAB3665) at 8 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Mouse IgG Secondary Antibody (red; Catalog # NL007) and counterstained with DAPI (blue). Specific staining was localized to nuclei. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.CDX2 in Human Colon Cancer Tissue.
CDX2 was detected in immersion fixed paraffin-embedded sections of human colon cancer tissue using Mouse Anti-Human CDX2 Monoclonal Antibody (Catalog # MAB3665) at 5 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Mouse IgG VisUCyte™ HRP Polymer Antibody (Catalog # VC001). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to nuclei. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.Detection of Human CDX2 by Immunocytochemistry/Immunofluorescence
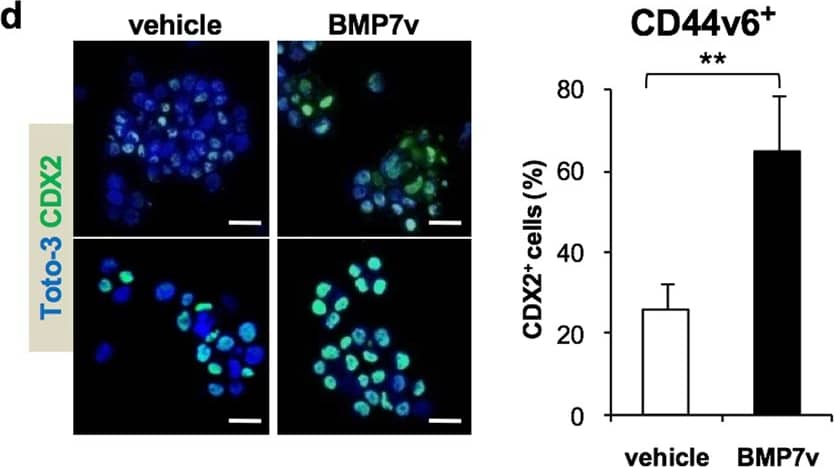
BMP7v treatment promotes CR-CSC differentiation. a Phase-contrast microscopy analysis of CD44v6+ CRC sphere cells treated with BMP7v at the indicated time points. One representative of CSC#1, 2, 4, 5, 7, and 23–26 is shown. The scale bar represents 20 µm. b Percentage of CK20 positive cells in CD44v6+ CR-CSCs treated with vehicle or BMP7v up to 21 days evaluated by immunofluorescence analysis. Data are expressed as mean ± SD of experiments performed in 15 CRC sphere cell lines (CSC#1–3, 5–7, 10,11, 14–16, 18, 25, 33, and 40). c Flow cytometry analysis of CD133/CD44v6 on CRC sphere cells treated with vehicle or BMP7v for 14 days. Data reported are mean ± SD of 15 CRC sphere cell lines analyzed (CSC#1–8, 10,11, 14–16, 18, and 25). d (left panels) Immunofluorescence analysis of CDX2 on CR-CSCs upon 14 days of BMP7v treatment. One representative of CSC# 3, 9, and 21 is shown. Nuclei were stained with Toto-3 (blue color). The scale bars represent 20 µm. (right panel) Percentage of CDX2 positive cells in CD44v6+ CR-CSCs treated with vehicle or BMP7v up to 14 days evaluated by immunofluorescence analysis. Data are expressed as mean ± SD of experiments performed in CSC# 3, 9, and 21. e Flow cytometry analysis of TOP-dGFP or CD44v6 in enriched CD44v6+ sphere cells treated with BMP7v up to 14 days. One representative experiment of CSC#1, 2, 4, 7, and 10 is shown. f Phase-contrast microscopy analysis of TOP-dGFP CRC sphere cells grown in matrigel drops and treated with vehicle, BMP7v or FBS for 14 days. One representative of CSC# 8, 9, and 11 is shown. The scale bar represents 100 µm. g Immunofluorescence analysis of E-cadherin, vimentin, and beta-catenin (green color) in CD44v6+ CRC cells exposed to vehicle or BMP7v for 14 days. One representative experiment performed in cells as in e is shown. Nuclei were stained with Toto-3 (blue color). The scale bars represent 20 µm. h Migrating CD44v6+ and CD44v6− cells treated with vehicle or BMP7v up to 48 h. Data are shown as mean ± SD of three independent experiments performed in five CRC sphere cell lines (CSC#1, 5, 7, 10, and 12). i Cell viability percentage of enriched CD44v6+ and CD44v6− cells treated with vehicle or BMP7v up to 96 h. Data are shown as mean ± SD of different experiments performed in CSC#1, 2, 4, 7, and 10. j Cell cycle analysis in CD44v6+ CR-CSCs exposed to vehicle or BMP7v for 72 h. The data show percentage of cell number in sub-G0, G0/G1, S, and G2/M phases. Data are expressed as mean ± SD of three independent experiments performed in five different CRC sphere cell lines as in e. k Immunoblot analysis of PARP, cleaved PARP (cPARP), Caspase-3 (Casp-3), cleaved Caspase-3 (cCasp-3), Bcl-2, Bcl-xL in CD44v6+, and CD44v6− enriched cells treated as in e for 72 h. beta-actin was used as loading control. One representative experiment performed in three different CRC sphere cell lines (CSC#1, 4, and 7) Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31591478), licensed under a CC-BY license. Not internally tested by R&D Systems.Applications for Human CDX2 Antibody
Immunocytochemistry
Sample: Immersion fixed SW480 human colorectal adenocarcinoma cell line
Immunohistochemistry
Sample: Immersion fixed paraffin-embedded sections of human colon cancer tissue
Formulation, Preparation, and Storage
Purification
Reconstitution
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: CDX2
Human CDX2 (Caudal type homeobox transcription factor 2) is a 37 kDa member of the caudal family of homeobox proteins. It is 313 amino acids (aa) in length and contains a 167 aa caudal-like activation domain (aa 13‑180), a DNA-binding homeodomain (aa 186‑245) and two short, poly-glutamine and -proline repeats. CDX2 can exist as a dimer and is regulated by phosphorylation of a 4(x)serine motif that promotes CDX2 degradation. CDX2 promotes intestinal patterning and development. While it is necessary but not sufficient for intestinal phenotype differentiation, it will block cell proliferation on its own. Over the amino acid range used for immunization, human CDX2 is 95% and 97% aa identical to mouse and canine CDX2, respectively.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional CDX2 Products
Product Documents for Human CDX2 Antibody
Product Specific Notices for Human CDX2 Antibody
For research use only