The Kynurenine Pathway and Tumor Progression
Tryptophan Metabolism Can Inhibit Immune Cell Functions
Tryptophan is an essential amino acid produced by the human body that is primarily metabolized by the kynurenine pathway, which plays a role in regulating the activities of different immune cell types.1 Indoleamine-2,3-dioxygenase (IDO1 or IDO2) and tryptophan-2,3-dioxygenase (TDO2) are key enzymes that catalyze the initial and rate-limiting step in this pathway. The activities of these enzymes convert L-tryptophan to N-formyl-kynurenine, which is then further converted into kynurenine. This impacts the immune system first, by depleting tryptophan from the local microenvironment, which inhibits T cell proliferation and activity,2-5 and second, by producing kynurenine, a potent immunoregulatory molecule, which inhibits T cell and natural killer cell proliferation and activity, and promotes the differentiation of regulatory T cells.6-9
Indoleamine-2,3-dioxygenase and tryptophan-2,3-dioxygenase are expressed by a variety of cell types including stromal, vascular, tumor, and immune cells, most notably on antigen-presenting cells.10 Additionally, indoleamine-2,3-dioxygenase expression is strongly induced by inflammatory molecules such as IFN-gamma, TNF-alpha, and IL-6, and multiple tumor cell types have been shown to express indoleamine-2,3-dioxygenase, tryptophan-2,3-dioxygenase, or both enzymes.10-12 High level expression of indoleamine-2,3-dioxygenase by tumor cells correlates with the expansion, recruitment, and activation of myeloid-derived suppressor cells (MDSCs), tumor aggressiveness, and resistance to immune checkpoint blockade.13 As a result, the kynurenine pathway and specifically small molecule inhibitors of indoleamine-2,3-dioxygenase are being investigated by immuno-oncology researchers as potential immunotherapeutic drugs.
Bio-Techne offers a range of R&D Systems enzymes in the kynurenine pathway along with Tocris® small molecule inhibitors of some of these enzymes for immuno-oncology research.
Kynurenine Pathway Enymes - Products by Molecule
Tryptophan Metabolism by the Kynurenine Pathway Can Inhibit Immune Cell Functions

High level expression of indoleamine-2,3-dioxygenase and tryptophan-2,3-dioxygenase by tumor cells or cells in the tumor microenvironment inhibits the functions of T cells and natural killer (NK) cells. Indoleamine-2,3-dioxygenase and tryptophan-2,3-dioxygenase, two enzymes involved in the metabolism of tryptophan, are produced by tumor cells or other cells present in the tumor microenvironment, including tumorassociated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs) and antigen-presenting cells (APCs). High level expression of indoleamine-2,3-dioxygenase and tryptophan-2,3-dioxygenase contribute to a favorable environment for tumor progression as they increase the rate of tryptophan degradation, leading to the depletion of tryptophan from the tumor microenvironment and the production of the immunosuppressive metabolite, kynurenine. Together, these conditions suppress T cell and natural killer (NK) cell proliferation and activity and promote the generation of regulatory T cells.
Comparison of the Enzymatic Activity of R&D Systems Recombinant Human IDO2 and a Competitor's IDO2 Protein
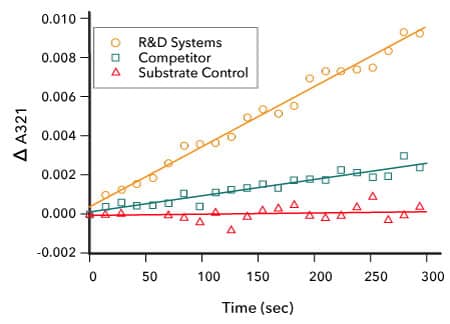
Measurement of IDO2 Enzyme Activity. The activity of Recombinant Human IDO2(R&D Systems, Catalog # 9967-AO) was measured by its ability to oxidize L-tryptophan to N-formyl-kynurenine. The activity of the R&D Systems IDO2 enzyme (orange) was found to be approximately 4-fold greater than a competitor’s IDO2 (green). The negative control is shown in red.
Comparison of the Enzymatic Activity of R&D Systems Recombinant Human TDO2 and a Competitor's TDO2 Protein

Measurement of TDO2 Enzyme Activity. The activity of Recombinant Human TDO2 (R&D Systems,Catalog # 9768-TD) was measured by its ability to oxidize L-tryptophan to N-formyl-kynurenine. The activity of the R&D Systems TDO2 enzyme (orange) was approximately 10-fold greater than a competitor’s TDO2 (green). The negative control is shown in red.
-
Erhardt, S. et al. (2009) Pharmacological manipulation of kynurenic acid: potential in the treatment of psychiatric disorders. CNS Drugs 23:91. PMID: 19173370.
-
Munn, D.H. et al. (1999) Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 189:1363. PMID: 10224276.
-
Mellor, A.L. et al. (2002) Cells expression indoleamine 2,3-dioxygenase inhibit T cell responses. J. Immunol. 168:3771. PMID: 11937528.
-
Munn, D.H. & A.L. Mellor (2007) Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J. Clin. Invest. 117:1147. PMID: 17476344.
-
Lee, G.K. et al. (2002) Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology 107:452. PMID: 12460190.
-
Frumento, G. et al. (2002) Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 196:459. PMID: 12186838.
-
Terness, P. et al. (2002) Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J. Exp. Med. 196:447. PMID: 12186837.
-
Della Chiesa, M. et al. (2006) The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood 108:4118. PMID: 16902152.
-
Yan, Y. et al. (2010) IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J. Immunol. 185:5953. PMID: 20944000.
-
Ye, Z. et al. (2019) Role of IDO and TDO in cancers and related diseases and the therapeutic implications. J. Cancer 10:2771. PMID: 31258785.
-
Marin-Acevedo, J.A. et al. (2018) Next generation of immune checkpoint therapy in cancer: new developments and challenges. J. Hematol. Oncol. 11:39. PMID: 29544515.
-
Burugu, S. et al. (2017) Emerging targets in cancer immunotherapy. Semin. Cancer Biol. 52:39. PMID: 28987965.
-
Holmgaard, R.B. et al. (2015) Tumor-expressed IDO recruits and activates MDSCs in a Treg-dependent manner. Cell Rep. 13:412. PMID: 26411680.