Metastasis
Metastasis, a major determinant of cancer fatality, is a complex, multistep and multifactorial process which often allows the dissemination of cancer cells from a primary tumor to distant tissues and organs leading to the establishment of secondary tumors. The metastatic process is first facilitated by cellular proliferation within primary lesions which results in tumor invasion through adjacent epithelium and basement membranes. This initial phase ultimately allows cells that detach from the primary tumor to migrate to nearby tissues or into blood and lymphatic vessels to reach secondary tissues where they may adhere and grow.

Molecules halting metastatic growth may act via a broad range of mechanisms including effects on cell adhesion properties, signaling pathways important for cell motility and survival, and transcription of genes with pro-metastatic potential. Metastasis suppressors, such as E-Cadherin and NDRG1 and TIMPs, prevent the establishment of cancer growth in secondary tissues without affecting growth of primary tumors.
The ability of primary tumor cells to become invasive is partly determined by the intrinsic expression and function of metastasis promoters and suppressors. The genetic basis of metastasis has been a key focus of cancer research leading to the identification of over 30 metastasis suppressors which are defined by their ability to prevent cancer spread without affecting primary tumor growth. However, extrinsic factors such as the tumor microenvironment have increasingly gained attention due to the crucial role of stromal cells and derived factors in the establishment of secondary tumors. Metastatic potential is not only influenced by the primary tumor’s microenvironment, but is also dependent on the cells and factors present at secondary targeted tissues.
Validated Metastasis Suppressors
|
Primary Tumor Site |
Metastasis Suppressor |
Main Secondary Tumor Site* |
|---|---|---|
|
Breast |
bone, brain, liver, lung, regional lymph nodes |
|
|
Colon |
liver, lung, peritoneum |
|
|
Lung |
adrenal gland, bone, brain, liver, lung |
|
|
Melanoma |
bone, brain, liver, lung, skin, muscle, lymph nodes |
|
|
Pancreas |
liver, lung, peritoneum |
|
|
Prostate |
adrenal gland, bone, liver, lung |
*Learn more about secondary tumor sites: https://www.cancer.gov/types/metastatic-cancer
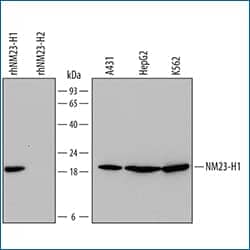
Western blot shows lysates of A431 human epithelial carcinoma cell line, HepG2 human hepatocellular carcinoma cell line, and K562 human chronic myelogenous leukemia cell line. PVDF Membrane was probed with 0.5 µg/mL of Mouse Anti-Human NM23-H1 Monoclonal Antibody (Catalog # MAB6256) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # HAF007). For additional reference, recombinant human NM23-H1 and recombinant human NM23-H2 (10 ng/lane) were included. A specific band was detected for NM23-H1 at approximately 20 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 2.
|
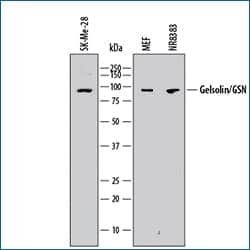
Western blot shows lysates of SK-Mel-28 human malignant melanoma cell line, MEF mouse embryonic feeder cells, and NR8383 rat alveolar macrophage cell line. PVDF membrane was probed with 0.5 µg/mL of Mouse Anti-Human/Mouse/Rat Gelsolin/GSN Monoclonal Antibody (Catalog # MAB8170) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # HAF018). A specific band was detected for Gelsolin/GSN at approximately 95 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1. |
Al-Mahmood, S., Sapiezynski, J., Garbuzenko, O. B., & Minko, T. (2018). Metastatic and triple-negative breast cancer: challenges and treatment options. Drug Delivery and Translational Research 8:1483–1507.
Brew, K., & Nagase, H. (2010). The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochimica et Biophysica Acta - Molecular Cell Research 1803; 55-71.
Budczies, J., von Winterfeld, M., Klauschen, F., et al. (2015). The landscape of metastatic progression patterns across major human cancers. Oncotarget 6:570-583.
Chen, L.L., Blumm, N., Christakis, N.A., et al. (2009). Cancer metastasis networks and the prediction of progression patterns. Br J Cancer 101:749–758.
Cook, L.M., Hurst, D.R., & Welch, D.R. (2010). Metastasis suppressors and the tumor microenvironment. Semin Cancer Biol 21(2):113-22.
Guan, X. (2015). Cancer metastases: challenges and opportunities. Acta Pharmaceutica Sinica. B. 5: 402-418.
Hunter, K.W., Crawford, N.P., & Alsarraj, J. (2008). Mechanisms of metastasis. Breast Cancer Res. 10 Suppl 1 S2.
Hurst, D. R., & Welch, D. R. (2011). Metastasis suppressor genes. At the interface between the environment and tumor cell growth. International Review of Cell and Molecular Biology 286:107-80.
Yan, J., Yang, Q., & Huang, Q. (2013). Metastasis suppressor genes. Histol Histopathol. 28: 285-292
Yokota, J. (2000). Tumor progression and metastasis. Carcinogenesis 21: 497–503.