Hypoxia Inducible Factors (HIFs)
HIF-1 and -2 alpha | HIF-3 alpha | HIF Regulation
Hypoxia-inducible factors (HIFs) are transcription factors that respond to decreased oxygen level in the cellular environment. Various isoforms and splice variants have been identified and their activities vary according with their specific expression patterns and structural domain composition.
HIF-1 and -2 alpha
Hypoxia inducible factor 1 (HIF-1) was first identified in the early 1990s and molecularly characterized as a heterodimer consisting of an alpha (oxygen-sensitive) and beta (oxygen-insensitive) subunits. Two additional isoforms, HIF-2 alpha and HIF-3 alpha, were subsequently identified. HIF-1 alpha and HIF-2 alpha are generally differentially expressed, but share several transcriptional targets with an overall positive response to hypoxia. Lastly, HIF-3 alpha is less well studied but is known to have both positive and negative effects on hypoxia responses.
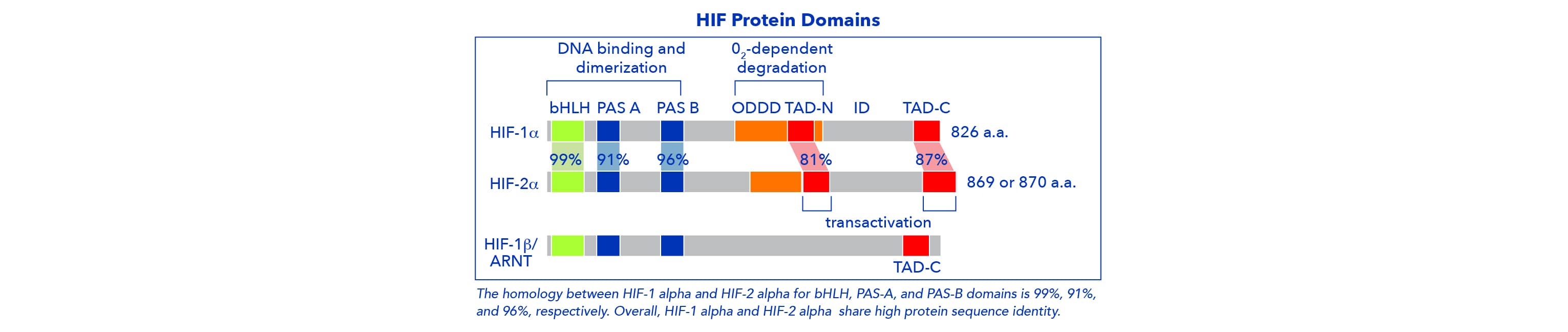
HIF-beta subunits are constitutively expressed, independently of oxygen levels, and present in the nucleus. HIF-alpha subunits are expressed constitutively, and predominantly regulated post-translationally. Recently, epigenetic mechanisms have been shown to regulate the transcription of HIF-1 alpha and HIF-2 alpha in response to hypoxia.
Expression Patterns of Major HIF – alpha Subunits |
|
| HIF-1 | Broad expression |
| HIF-2 | Tissue specific expression (e.g., in specific cell types within the lungs, liver, brain and pancreas) |
| HIF-3 | Expressed widely in tissues (e.g., placenta, lung, liver, heart, kidney, cartilage, brain and muscle). Spliced variants have tissue-specific expression. |
The HIF-alpha subunit isoforms form heterodimers with HIF-1 beta (aryl hydrocarbon receptor nuclear translocators- ARNT) or HIF-2 beta (ARNT2), and as a complex regulate gene expression.
HIF-3 alpha
Alternative splicing of HIF-3 alpha mRNA and multiple transcriptional start sites within the HIF-3 alpha gene result in several mRNA products and protein variants. Some of these products completely lack transactivation domains and are unable to regulate gene expression. HIF-3 alpha subunits without TAD, also known as PAS domain proteins (IPAS), act as inhibitors of both HIF-1 and HIF-2 activity. HIF-3 isoforms with an TAD-N sequence are able to regulate gene expression under hypoxia. Because all HIF-3 isoforms contain bHLH and PAS domains, variants form heterodimers with HIF- beta subunits.
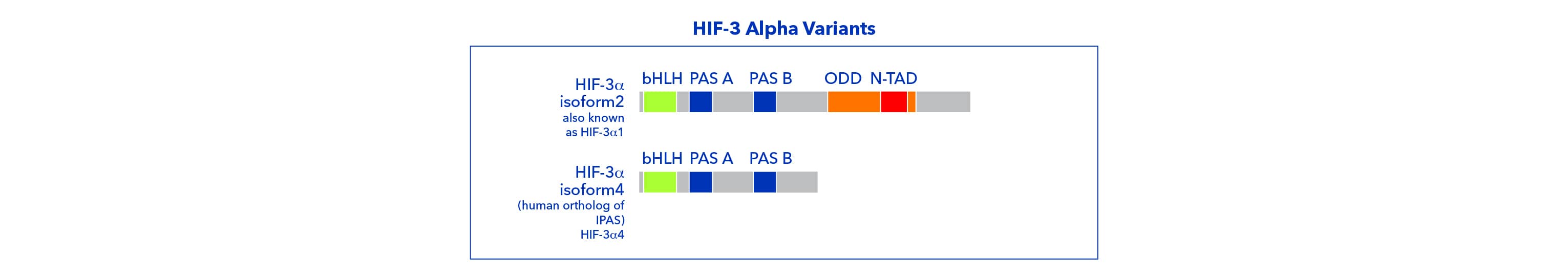
HIF-3 alpha transcripts are broadly expressed in human tissues and organs, with some variants present at higher levels at embryonic stages. Many HIF-3 alpha variants contain an ODDD sequence, and therefore protein stabilization under hypoxia is expected. However, HIF-3 alpha is known to be regulated via mechanism that target its transcription, translation and nuclear localization.
Factors Regulating HIF-3 Expression |
|
|
HIF-1 alpha, |
Transcriptional induction of HIF-3 alpha via a hypoxia responsive element (HRE) upstream of Exon 1b |
|
Induction of HIF-3 alpha isoform 4 or IPAS expression |
|
|
miR-485-5p, |
Inhibition of HIF-3 alpha expression |
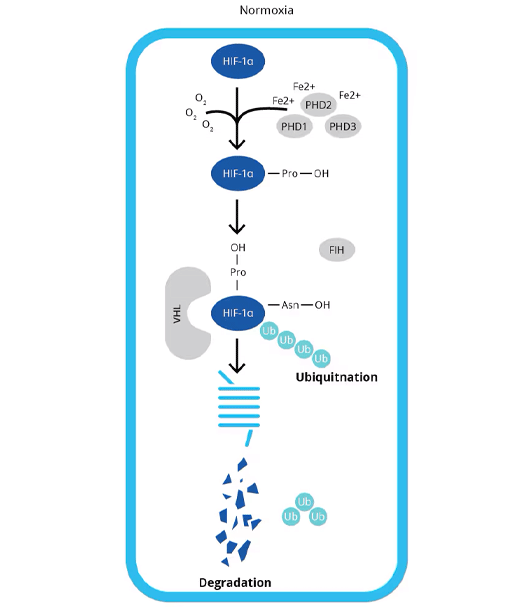
Normoxia: Under normoxia, HIF-alpha subunits are short-lived, with a half-life of less than 5 minutes, due to ubiquitin-dependent proteasomal degradation. This process is initiated by PHDs, which catalyze the post-translational hydroxylation of specific proline residues within the oxygen-dependent degradation domain (ODDD) of HIF-alpha subunits. Binding of hydroxylated HIF-alpha subunits to the von Hippel-Lindau protein (pVHL) recruits the E3 ubiquitin ligase system and leads to the degradation of HIF-alpha by the 26S proteasome.
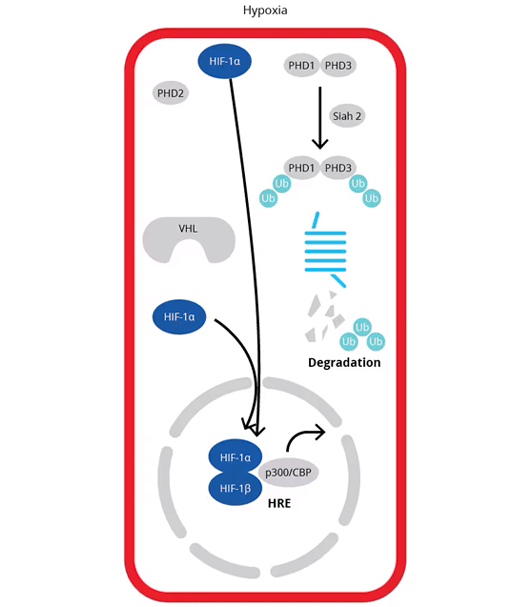
Hypoxia: Under hypoxia, proline-hydroxylation of the HIF-alpha subunit by PHDs is inhibited. PHDs are targeted for degradation by E3 ubiquitin ligases Siah1a and Siah2, preventing the association of HIFs with pVHL . Stabilization of HIFs occurs under hypoxic conditions because PHDs are inactivated, and HIFs escape ubiquitination and degradation. Stabilized HIF-alpha freely moves to the nucleus where it forms a heterodimer with HIF-beta and by interacting with co-activators p300/CBP regulates the expression of target genes containing the promoter core sequence [A/G]CGTG or hypoxia responsive element (HRE).
Major Enzymes Regulating HIF-1 alpha
Regulators of HIF-1α |
Regulatory Mechanisms |
|
| Negative | Casein kinase 1 (CKI) | Phosphorylation within PAS-B (Ser-247) destabilizes the HIF-1α-ARNT complex and reduces transactivation |
| PHDs | Hydroxylation within ODDD (Pro-402) and TAD-N (Pro 564) lead to reduced stability of HIF-1α | |
| FIH | Hydroxylation and TAD-C (Asn-803) causes faulty transcriptional activation by HIF-1α | |
| Glycogen synthase kinase 3β (GSK3β) | Phosphorylation within ODDD (Ser-551, -555, -588) results in diminished stability of HIF-1α | |
| Positive | ERK1 | Phosphorylation within C-terminal domain (Ser-641, -643) masks a nulear export signal and results in nuclear accumulation and enhanced activity of HIF-1α |
| Protein kinase A (PKA) | During intermittent hypoxia, PKA functions like ERK1 | |
| p300/CBP-associated factor (PCAF) | Acetylation within C-terminal domain (Lys-674) leads to increased HIF-1α levels and binding of p300 | |
| p300 | Acetyl-transferase activity of p300 within C-terminal domain (Lys-709) blocks ubiquitination-induced degradation of HIF-1α | |
| Casein kinase II (CKII) | Hypoxia-induced phosphorylation within TAD-C (Thr-796) decreases affinity of HIF-1α for FIH |